Researchers from the Dana-Farber Cancer Institute have developed a new blood test that would accurately determine the presence of several types of cancers. The results of their multi-centre clinical trial to prove the effectiveness of their test was presented by the researchers at the European Society for Medical Oncology (ESMO) 2019 Congress in Barcelona, Spain, on the 28th of September 2019.
The test developed by GRAIL, Inc. called the “next-generation sequencing (NGS) blood test” is a unique test that uses genetic sequencing as a tool to discover the abnormal DNA and their chemical tags or methyl tags. These tags could reveal if the genes are active or inactive, write the researchers. For this study titled, “Simultaneous multi-cancer detection and tissue of origin (TOO) localization using targeted bisulfite sequencing of plasma cell-free DNA (cfDNA),” around 3600 blood samples were tested. Some of these blood samples came from patients who were already diagnosed with cancer while others were from persons who did not have cancer. The test could successfully and accurately determine the individuals who had cancer and also detected the “tissue of origin” or TOO for the patient. TOO meant that organ that was afflicted by the cancer.
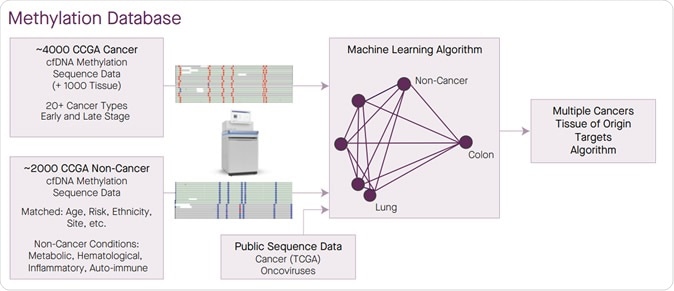
A large methylation sequence database of cancer and non-cancer was generated to enable target selection for a single test able to classify multiple cancers at high specificity and identify TOO.
There are two markers for a test – its sensitivity and specificity. Specificity of a test means that it would show a positive result only when the cancer was actually present, and sensitivity meant it would yield a negative result only when the cancer was absent in a sample. The researchers noted that this new test by GRAIL was highly specific and could also detect the organ from which the cancer had originated.
The specificity of the test was found to be 99.4 percent meaning that the test returned with a false positive result in only 0.6 percent of cases. Sensitivity of the test showing actual samples that tested positive for cancer among the total samples was 76 percent. Sensitivity was 32 percent for patients with Stage I cancer, 76 percent, 85 percent and 93 percent respectively for stages II, III and IV cancers. For all types of cancers, the sensitivity was 55 percent. The test revealed TOO or type of cancer in 98 percent cases of which 89 percent were correct.
The basis of the test was to detect the DNA from the cancer cells that spread in the bloodstream of the patient after the cells die. These blood samples thus carry the dead cancer cells from which the test can detect the DNA abnormalities. The researchers called this “liquid biopsies” that could help detect not only genetic mutations of the cancer cells but also alterations seen in the cellular DNA due to the cancer.
The team wrote that there are certain methyl groups that are attached to the DNA by a process called methylation. These methyl groups are chemical units that can control the on and off position of the genes. Their abnormalities lead to indicators of cancers, explain the researchers. This new test looks at regions of the DNA that shows abnormal pattern of methylation, write the researchers.
Lead author, Geoffrey Oxnard, MD, of Dana-Farber, in a statement said, “Our previous work indicated that methylation-based assays outperform traditional DNA-sequencing approaches to detecting multiple forms of cancer in blood samples. The results of the new study demonstrate that such assays are a feasible way of screening people for cancer.” The team used this test to analyse the free DNA in the blood that has come from the dead cancer cells. These are termed “cell-free DNA”. The results came from 3,583 blood samples of which 1,530 were from patients with cancer and 2,053 from persons without cancer. Among the samples with cancer, there were over 20 different of types of cancers including breast cancer (hormone receptor negative type), food pipe or esophageal cancer, gallbladder, head and neck, gastric, colorectal, lymphoid leukemias, lung, multiple myeloma, pancreatic and ovarian cancers the team wrote.
Anne-Renee Hartman, MD, Vice President of Clinical Development at GRAIL, in a statement said, “Most cancers go undetected until too late, and cancer remains the second leading cause of death worldwide. To address this challenge, we embarked on one of the most ambitious clinical study programs in genomic medicine in support of a novel multi-cancer approach to early cancer detection.” “We are pleased to be presenting new data from our large-scale, rigorous clinical study program that demonstrate the ability of our test to detect more than 20 cancer types across all stages from a single blood draw and identify where the cancer is located in the body, while minimizing the rate of false positives,” she added.
GRAIL will be presenting additional data from the related studies at the in Bangkok, Thailand between 11th and 13th October 2019.