A new study published in the journal Oncotarget shows that certain mutations in the brain tumor called meningioma may help identify patients who have a better chance of recovery. This could help physicians make better clinical decisions based on more accurate diagnoses. It has also unveiled some of the genes that go wrong in meningiomas, as well as showing how targeted therapy could one day help fight this tumor.
Meningiomas are tumors arising from the meninges, or brain coverings. They are the most common form of brain cancer in adults, occurring in 170,000 adults in the US. Most of them arise from the arachnoid mater, and the majority are benign. However, about one in five are aggressive, and in grade II or III, the recurrence rate is 50% to 80%. Thus understanding what drives the aggressiveness of these tumors is important in evolving better therapies.
They are usually treated by complete surgical resection. However, this is difficult for tumors at the base of the skull, which also holds multiple vital structures such as the medulla, the brain stem, the spinal cord and the posterior ventricle very near the tumor.
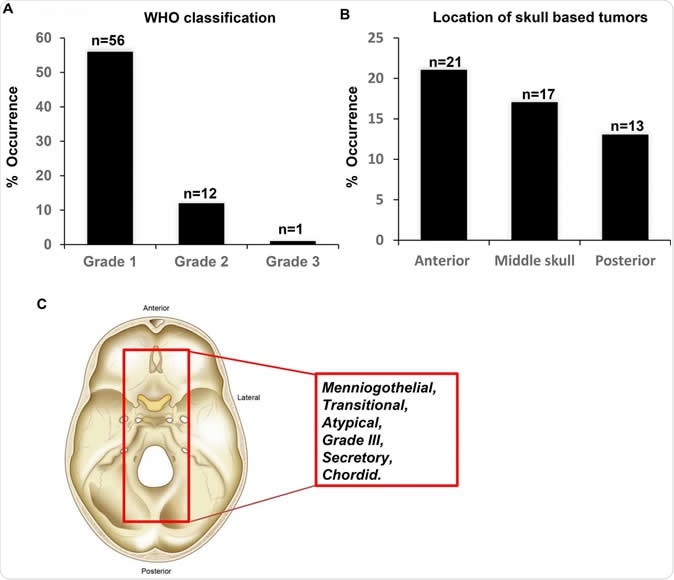
Figure 1: Characteristics of the meningioma's investigated in the study. (A) Tumor grade according to the most recent WHO classification. (B) Location of the skull based tumors. (C) Schematic demonstrating the histology of the identified tumors from the study cohort. Image Credit: Malak Abedalthagafi
Grades of meningiomas
At present, different types of meningiomas are distinguished only by their histology, since the genetic mutations that stimulate or promote the formation of these tumors are not properly understood. With respect to the histology, or tissue structure, the WHO classification has grade I–III based on the presence of nuclear mitoses, invasive characteristics and other signs of aggressive behavior. Grade II meningiomas are those which show the cell types called clear cell or chordoid forms. Grade III are those which show papillary formation.
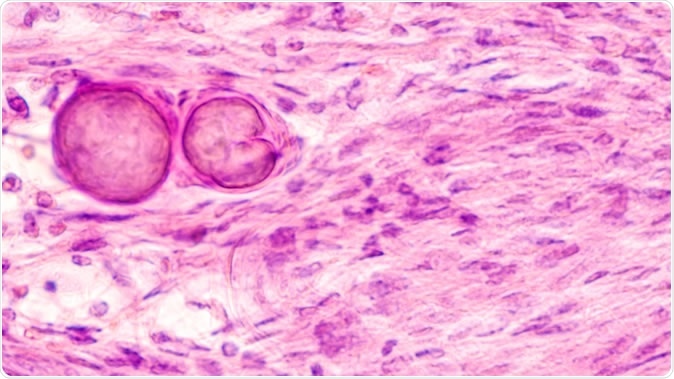
Stereotactic brain biopsy smear cytology of a meningioma, a benign brain tumor of the meninges, showing fascicles of spindle cells with psammoma bodies. - Image Credit: David A. Litman / Shutterstock
It is known that neurofibromin 2 is the primary tumor suppressor gene implicated in meningioma, and this gene is found in 40% to 60% of tumors in the early stages. In some studies carried out on primary brain tumors in Saudi Arabia, the most common histological type is found to be a grade I meningioma. These tumors recur in 11-22% of patients. Mutations such as NF2 and MN1 have been proposed to be driver mutations which enhance the aggressive development of the tumor and make it more likely to spread fast and widely.
The study and its outcomes
The current study looked at the genomes of the cancer cells in 71 meningioma patients diagnosed between 2008 and 2016. Among these, 51 were skull base meningiomas. A next-generation DNA sequencing technique was used to identify a custom cancer gene array, using targeted sequencing methods.
The researchers found multiple mutations in these tumors, about 1.6 genomic alterations on average per tumor. The greatest frequency of mutation was for NF2 genes, seen in just over half of the cases. 80% of NF2 tumors are in the skull vault. NF2 mutations make the genome more unstable compared to non-NF2 mutations. On the other hand, the recurrence rate with non-NF2 mutated tumors was significantly higher, almost threefold, compared to those carrying NF2 mutations. This implies that genetic testing should go beyond a narrow range of NF2 testing to obtain clinically relevant information.
In non-NF2 genes too, there were a number of mutations, among which one was a hotspot TERTp c.–124 G>A mutation that seems to be linked to a poor prognosis. The PI3KCA gene is the most frequently mutated gene in non-NF2 tumors, and typically such tumors resist treatment. They also found mutations in the Fibroblast growth receptor-3 (FGFR3) genes that could forecast a better response to treatment. This was the first time these mutations have been identified in meningiomas occurring at the base of the skull.
Conclusion
The current study found several new mutations in skull base meningiomas lacking NF-2 markers, and the authors postulate that these may influence the prognosis of the tumor. While FGFR3 mutations are present in several malignancies, including those of the breast, prostate, bladder and squamous non-small cell lung carcinoma, these have been identified in this tumor for the first time. The interesting thing is that in other organs these mutations are usually linked to low-grade cancers, which have a low recurrence rate and a good outcome. Thus patients with these mutations typically have grade I tumors and in the current study, none of the three patients with FGFR3 mutations had a recurrence.
These initial findings show that more research is required to study genomic alterations in a range of meningiomas of different grades, to clarify their significance with respect to the outlook for tumor treatment and recurrence, and ultimate prognosis.
Journal reference:
New insights into the genomic landscape of meningiomas identified FGFR3 in a subset of patients with favorable prognoses. Aysha Al Sahlawi, Rasha Aljelaify, Amna Magrashi, Mariam Al Saeed, Amal Almutairi, Fatimah Alqubaishi, Abdulellah Alturkistani, Abdullah Al Obaid, Mohamed Abouelhoda, Latifa Al Mubarak, Nada Al Tassan and Malak Abedalthagafi. Oncotarget. 2019; 10:5549-5559. https://doi.org/10.18632/oncotarget.27178. http://www.oncotarget.com/index.php?journal=oncotarget&page=article&op=view&path%5B%5D=27178&path%5B%5D=87171