Skin cancer, particularly melanoma, which is the deadliest and most serious type of human skin cancer, begins as a small lesion or blemish. Usually, these blemishes start off as harmless accumulation of melanocytes, which give the skin its color. As the disease progresses, it can spread throughout the body.
A team of scientists at the University of Texas in Austin are now using supercomputers and algorithms to reveal the mechanism that stimulates cell mutations found in about half of all cases of melanoma.
Skin cancer is the most common cancer in the United States and across the globe. It is expected that about one in five people in the country will develop skin cancer when they reach the age of 70 and more than two people die from skin cancer in the U.S. every hour.
Though melanoma is the least common type of skin cancer, it is the deadliest. In 2019, it is projected that there will be about 96,480 new cases of the skin cancer in the United States, and about 7,230 people will die from the illness.
Skin cancer and B-Raf mutation
Researchers found an association of skin cancer with certain mutations of B-Raf (Rapidly Accelerated Fibrosarcoma) kinase, which is a protein that is part of the signal chain that begins outside the cell and goes inside to trigger growth of the cell. The Ras/Raf/Mek/Erk kinase pathway is vital for research of cancer, looking for answers to further understand how cells grow out of control. The study revealed that an estimated half of melanoma cases have a particular single mutation on B-Raf, dubbed as the valine 600 residue to glutamate (V600E).
The mutation has since become a crucial target of various drugs and through the years, certain inhibitors of the mutation were developed. These drugs used to inhibit the mutation along the way, a strange event occurred. Inhibiting the mutation has a disadvantage, since it stimulated the unmutated, wild type B-Raf protein kinases, which again triggered the development of the deadly skin cancer.
"With this background, we worked on studying the structure of this important protein, B-Raf. We aimed to study the more native-like state of the protein to understand how it's regulated in the cells, because most of the studies have been focused on the isolated kinase domain and how the drugs bind to the kinase domain,” Yasushi Kondo, a postdoctoral researcher in the John Kuriyan Lab at UC Berkeley and a co-author of the study, said.
Yin and yang circular symbol
The researchers compared the B-Raf dimer to the yin and yang circular symbol. They used computer models and simulations to confirm a valid finding. They conducted molecular dynamic simulations of the B-Raf dimer to test its stability of the asymmetric conformation. The team isn’t sure why the conformation was asymmetric or imbalanced, or what is aims to do in the maintenance of the enzyme’s active state.
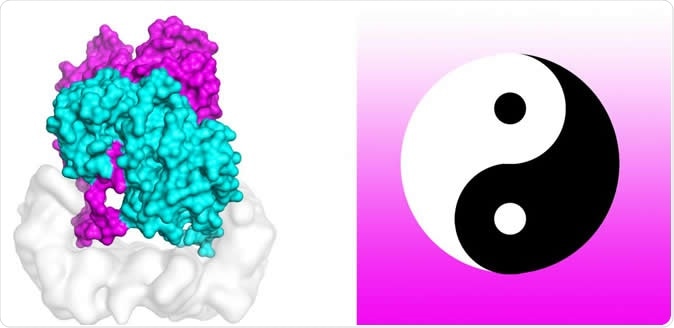
Scientists are using powerful supercomputers to uncover the mechanism that activates cell mutations found in about 50 percent of melanomas. Molecular dynamics simulations on TACC's Stampede2 supercomputer tested the stability of the structure of B-Raf:14-3-3 complex, which when mutated is linked to skin cancer. The study authors compare the B-Raf dimer to the Chinese yin-yang circular symbol of interconnected opposites joined at the tail. Image Credit: Karandur et al., TACC
They found that in the system that has no distal tail segment, the complex was entirely unstable. Another main finding of the study shows is discovering the mechanism of action that turns on the B-Raf kinase complex of two B-Raf kinases and two 14-3-3 scaffolding proteins, wherein B-Raf kinase is the stimulator or activator, while the other is the receiver.
The researchers admitted that their study has limitations. For one, small systems provide quick observational results while in large systems, certain changes occur in nanosecond, microsecond, and millisecond timescales. The Stampede2 supercomputer performed all simulations and it provided fast and efficient results.
The new study will pave the way for future development of new treatments and therapies for melamoma. The study was published in the American Association for the Advancement of Science’s journal, Science.
Journal reference:
Cryo-EM structure of a dimeric B-Raf:14-3-3 complex reveals asymmetry in the active sites of B-Raf kinases, Yasushi Kondo, Jana Ognjenović, Saikat Banerjee, Deepti Karandur, Alan Merk, Kayla Kulhanek, Kathryn Wong, Jeroen P. Roose, Sriram Subramaniam, John Kuriyan, Science 04 Oct 2019: Vol. 366, Issue 6461, pp. 109-115 DOI: 10.1126/science.aay0543, https://science.sciencemag.org/content/366/6461/109