A new trial involving the use of an antibody called anifrolumab reports improvement in patients with systemic lupus erythematosus (SLE) after 52 weeks of treatment. The research, titled 'Trial of Anifrolumab in Active Systemic Lupus Erythematosus', is published in the New England Journal of Medicine.
SLE is a debilitating autoimmune condition associated with a high level of illness and organ damage in the patient, including joint swelling, joint pain and skin lesions, as well as kidney disease. It is typically treated with glucocorticoids and immunosuppressants, to reduce the abnormal immune activity that triggers the systemic inflammation. However, one approved agent called belimumab can be used to treat this condition specifically.
Why only one agent for this long-established disease? The answer is that there is a host of genes expressed in SLE, giving rise to different biological mechanisms of disease and clinical features. However, the interferon family of cytokines, or cell signaling molecules that take part in inflammation, has been reported to be a potential target for therapy. Earlier trials have shown improvement with a specific anti-interferon therapy, using anifrolumab.
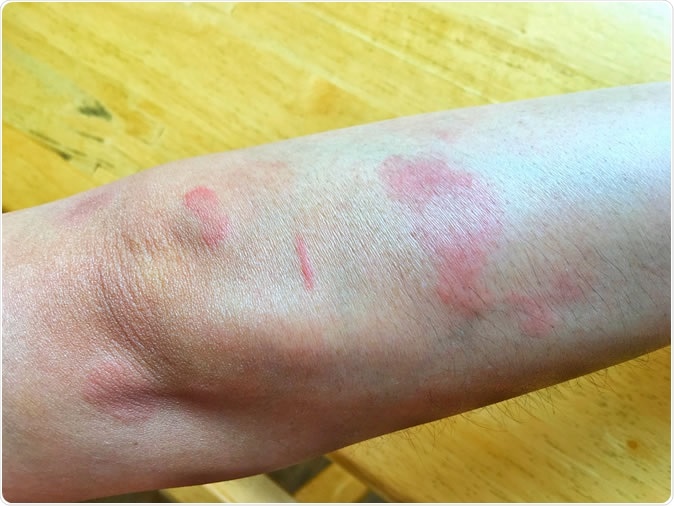
Systemic Lupus Erythematosus(SLE) is a chronic disease caused by self-immunity which causes Effects on the organs in the body, especially the skin, joints, blood, kidneys and central nervous system. Image Credit: Korn Ratchaneekorn / Shutterstock
Anifrolumab
Anifrolumab is a fully human IgG1κ monoclonal antibody that antagonizes the action of the type 1 interferon receptor subunit 1. By so doing, it prevents the action of all type 1 interferons. A previous trial called TULIP-1 (Treatment of Uncontrolled Lupus via the Interferon Pathway) showed it to be effective in SLE with respect to several end points, but the primary end point failed to show any significant effect. The end point here was a significant change in SRI (4) (Systemic Lupus Erythematosus Responder Index).
One of the secondary endpoints was the British Isles Lupus Assessment Group (BILAG)–based Composite Lupus Assessment (BICLA), and this showed a response favouring the use of anifrolumab. This was taken as the primary endpoint in the current trial, called TULIP-2.
The study
The patients were between 18 and 70 years of age, and had a score of 6 or more on the Systemic Lupus Erythematosus Disease Activity Index 2000 (SLEDAI-2K) that assigns scores from 0 to 105, as well as a purely clinical score (without laboratory results) of 4 or more on the same scale. They also had severe or moderate disease in one or two organs at least, respectively, according to the BILAG-2004 index. All patients tested positive for antinuclear antibodies, anti-dsDNA antibodies, or anti-Smith antibodies. All were being treated with glucocorticoids or other anti-inflammatory or immunosuppressant drugs.
The patients were randomly assigned to receive either placebo or anifrolumab once in four weeks for 48 weeks. There were 181 patients in the anifrolumab group and 184 in the placebo group. Each group had equal representation of SLEDAI-2K scores below 10 or above 10, similar dosages of prednisone or equivalent drugs, and comparable type 1 interferon gene expression levels.
The endpoint was evaluated at week 52, as the percentage of patients with a reduction in severity of disease by BICLA BILAG-2004 scores at baseline, no intensifying of disease, no discontinuation of the treatment, and no use of other treatments except those allowed in the trial protocol.
The findings
A BICLA response was seen in 86/180 patients on anifrolumab and 57/182 on placebo. This corresponds to a response rate of about 48% and 32% respectively. In the subset of patients with high interferon activity at baseline, 48% in the group on anifrolumab and 31% of those on placebo responded similarly. The percentage of BICLA response in patients on anifrolumab and placebo with low interferon activity was similar, but with slightly higher response in the latter compared to the other two groups.
Over half of the patients on steroids were able to reduce their dosage long-term on anifrolumab, compared to 30% of those on placebo. Skin disease activity was reduced in 50% of those on anifrolumab but half this number in the group on placebo. Severe joint involvement was attenuated in almost similar numbers in the anifrolumab and placebo groups, with about 5 percentage points difference.
Interferon activity was neutralized in a high 83% of those on anifrolumab but not in the placebo group. Adverse events were present in almost equal numbers in both groups. Serious adverse events occurred in about 8% and 17% of patients on anifrolumab and placebo, respectively. One flare-up occurred in the anifrolumab group and 6 in the placebo group.
About 3% and 7% of patients discontinued the intervention due to adverse events in the anifrolumab and placebo groups. Herpes zoster occurred in 7% and 1% of these groups, respectively, all resolving without stopping the intervention. Bronchitis and upper respiratory infections were at least twice as frequent in the anifrolumab group, and one death occurred in this group due to pneumonia.
Conclusions
Anifrolumab is different from other antibodies against type 1 interferon in its mechanism of action which targets the common type 1 receptor subunit required for signaling by all the type 1 interferon subtypes.
Overall, the percentage of patients with a BICLA response was significantly greater in patients on anifrolumab, as well as those who showed a favorable response in 3 of 5 secondary end points. These included reduced skin lesions and reduced steroid dosage. The use of the BICLA response in the TULIP-2 trial was in order to capture the effects of partial improvement in organs affected by SLE, in contrast to the SRI which can only give a Yes/No score.
The trial shows that this antibody achieves improvement in the overall disease score compared to placebo when used over a year. More work will be needed to find out how the effect lasts beyond this time, and the risks associated with longer duration of therapy.
Journal reference:
Trial of Anifrolumab in Active Systemic Lupus ErythematosusE.F. Morand, R. Furie, Y. Tanaka, I.N. Bruce, A.D. Askanase, C. Richez, S.-C. Bae, P.Z. Brohawn, L. Pineda, A. Berglind, and R. Tummala, for the TULIP-2 Trial Investigators. The New England Journal of Medicine. doi: 10.1056/nejmoa1912196, https://www.nejm.org/doi/full/10.1056/NEJMoa1912196