Researchers have discovered the molecular origins of the heart beat. They have found the proteins that make up tiny sodium channels in the tissues of the heart that can help generate the heart beats. The results of the study titled, “Structure of the Cardiac Sodium Channel” was published yesterday 19th of December 2019 in the journal Cell.
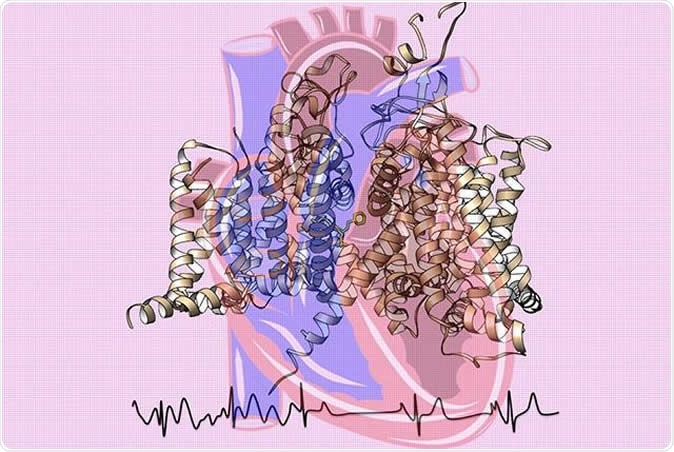
Cardiac sodium channel structure cartoon with binding of the antiarrhythmic drug flecainide shown as yellow sticks. The channel drawing is superimposed over a heart image. The electrocardiogram's chaotic atrial fibrillation signals (left) shift into a normal rhythm (right). Image Credit: Catterall and Zheng labs at UW Medicine
The team of researchers from the University Of Washington School Of Medicine explained that this is the first time that the generation of the electric signals within the heart has been described in such details. William Catterall and Ning Zheng, the senior authors of the study are both professors of pharmacology and the first authors of the study are Daohau Jiang and Hui Shi who are pursuing postdoctoral fellowships in pharmacology. They also added that this finding can lay the foundation for several studies that may help understand and diagnose heart rhythm disorders and also help develop drugs for these diseases.
Catterall said in a statement, “The cardiac sodium channel not only initiates the heartbeat, mutations in it also cause deadly arrhythmias, and antiarrhythmic drugs act directly on it to control cardiac rhythms.” The professors said that every heart beat generated in the sino atrial node of the heart is actually an electrical impulse. It can travel across the heart muscles in a coordinated manner so that the heart fills up with blood and is able to pump it out in synchrony. At the molecular level in the membranes of the cardiac cells there are tiny pores that regulate the movement of charged ions such as sodium, potassium, chloride etc. across the membranes.
This study reveals that sodium ions can pass through these pores made up of tiny proteins and connect the outside and inside of the cell membranes. They found that a toxin – puffer fish toxin (Tetrodotoxin), is capable of working on the sodium channels of the nerves and muscles but is incapable of stopping the sodium channels of the heart. Zheng explained, “Sodium channels operate in concert with calcium channels and potassium channels to drive the heartbeat at a consistent frequency for our entire lives.” He said that if these sodium channels do not function as they should there can be problems of heart rhythms that can sometimes be life threatening. They called these channels NaV (Latin abbreviation for sodium, V for voltage) 1.5 channel. They wrote that if the NaV channels were not formed correctly due to mutations, there could be life threatening arrhythmias leading to sudden deaths in children, adults and sportspersons.
Michael Lenaeus, a UW assistant professor of medicine, Division of General Internal Medicine, one of the authors of the study explained that one of the problems associated with abnormal rhythms is Atrial Fibrillation or A-Fib. He said that it affects many senior Americans. Drugs such as Flecainide are available for treating this condition, he explained.
For this study the team used a high-resolution, 3-D map of the channels made using advanced cryo-electron microscopy at the new Beckman Center for Cryo-EM at the UW. This map provided a clear picture of the channels and their functions. Now they could study the response of these channels to various drugs used against arrhythmias, poisons, toxins etc. they can also study the mutations of these channels and how it would affect the electrical activities within the heart.
This study provided a clear picture of the NaV1.5 channel and identified the key features that help differentiate it from other sodium channels. They also found the mutations and their effects on the heart muscles. They also tried to understand the Brugada syndrome there is a blockage of conductance of the sodium ions and how it could be diagnosed and treated effectively. They also looked at the effects of tetrodotoxin sensitive and insensitive sodium channels. The team also looked at the effects of delayed transmission and also the resting and activated states of these channels. They found that these heart sodium channels inactivate within 1 to 2 milliseconds of its rapid activation. This activation and rest was found to be important for the regular heart rhythm, they noted.
Authors wrote summarizing the results, “The structure of the cardiac sodium channel reveals key functional features. The antiarrhythmic drug flecainide blocks the pore below the selectivity filter. The ion selectivity filter and inactivation gate are revealed in atomic detail and an arrhythmia mutation creates a pathogenic gating pore ∼2 Å in diameter.”
Catterall said in conclusion, “Our high-resolution cryoEM structure of this iconic sodium channel gives new structure/function insights, reveals the molecular mechanisms of many inherited arrhythmias, and elucidates the exact binding site and blocking mode of the antiarrhythmic drug flecainide, which is used to treat atrial fibrillation, an increasingly prevalent problem in our aging population.” “Our results provide detailed insights into Na v1.5 structure, pharmacology, activation, inactivation, ion selectivity, and arrhythmias,” they wrote in conclusion.
This study was supported by the National Institutes of Health and the Howard Hughes Medical Institute.
Journal reference:
Structure of the Cardiac Sodium Channel Jiang, Daohua et al. Cell, https://www.cell.com/cell/fulltext/S0092-8674(19)31326-1