The process of developing new drugs is both difficult and costly. It is estimated that of all the drug candidates which start out on their testing journey, less than 14% actually make it to the clinical scene and receive approval by the US Food and Drug Administration (FDA). Animal studies are not only extremely cost-heavy and time-consuming on the drug development scale but are also becoming increasingly controversial as animal rights activists become more militant. These issues have made it a matter of great importance to find replacements for animal models.
One area of drug development that absolutely demands preclinical testing in animals is the study of the drug absorption, distribution, metabolism and excretion (ADME) within the body. This is called pharmacokinetics and is responsible for the final concentration of the drug in the body. Drug metabolism is affected by the signaling pathways between a host of organs through the vascular channels containing blood.
Animal testing is also essential for the study of drug pharmacodynamics (PD) where the effects of the drug on various organs are revealed. This area is important because it is responsible for the mechanism of action of the drug and the side effects. New research out of Harvard’s Wyss Institute for Biologically Inspired Engineering reports significant progress in shifting to a more ethical testing procedure while still maintaining reliability.Two back-to-back publications in Nature Biomedical Engineering, describe the Wyss team’s research in full.
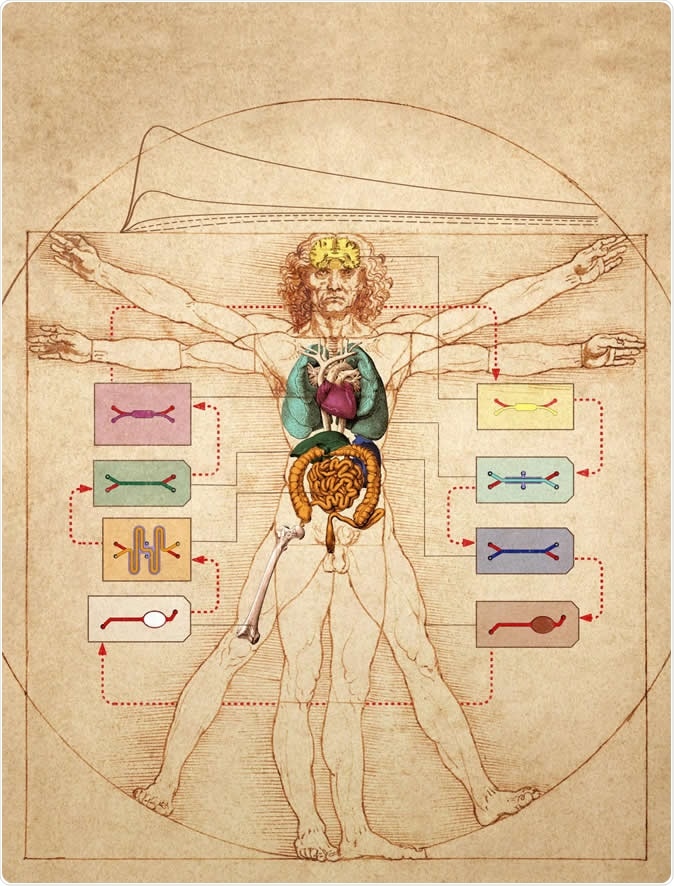
In this graphic, the Wyss Institute's human Body-on-Chip system is layered on top of Leonardo da Vinci's ink drawing of the "Vitruvian Man", which represents ideal human body proportions. The researchers used a computational scaling method to translate data obtained from drug experiments in the human Body-on-Chip to the organ dimensions of the real human body. Image Credit: Wyss Institute at Harvard University
Organ chips
An organ chip is a device that is designed for microfluidic culture. Built of clear flexible polymer, it is only as big as a USB drive, and consists of two channels running down its length in parallel, with a porous membrane partition between them. On one side, the channel contains cells from a specific organ. On the other side, there are vascular endothelial cells that grow in the embryonic pattern to recreate a blood vessel. Each channel is bathed with a medium specific for its cell type.
The presence of the porous partition allows signaling between compartments. Moreover, molecules like cytokines, growth factors, drug molecules and drug metabolites are all able to pass through, as they are formed during the specific metabolic activities that occur in each different organ.
The first organ chip, which was popularly called by the catchier slogan “Organ on a Chip”, was developed in 2010. This model recreated the normal functioning of the lung as well as the disease process, with incredible accuracy.
The body-on-chip concept
The current body-on-chip concept was proposed by study author Donald Ingber in 2011, based on the presence of the vascular endothelium-lined channel. The basic assumption was that if the working of one organ could be recreated, so could the entire body, by interlinking the vascular channels between multiple types of organ chips in the same way the organs in the body are connected through one common circulatory system. This would make it possible to evaluate both pharmacokinetics and pharmacodynamics across the miniature body-mimicking system rather than on different types of cells in vitro, which fall far short of the actual physiological arrangement.
This concept gained power from the realization of the many shortcomings of the experimental animal models of today, including the delay involved, in a situation where biological agents of many different types pose a great threat to global health, requiring immediate response.
The reality
This enabled the project to obtain a grant from the Defense Advanced Research Projects Agency (DARPA) the next year for an extremely ambitious DARPA-dictated undertaking. This was nothing less than to create an artificial body-on-chip, consisting of 10 different human organs linked by an automated fluidic instrument. Exploiting the power of computational modeling, the scientists were expected to analyze the data obtained from this platform to predict how a given drug would affect the body and what level it would achieve in various dosages.
The success of the team in overcoming this major challenge and coming out with accurate results is described in two consecutive papers in the journal.
The platform
In the first paper, the modular body-on-chips platform is described. It centers on an ingenious interrogator device capable of culturing up to 10 different organ chips as well as robotically directed sequential fluid transfer between the endothelium-lined vascular channels, all linked to simulate the body’s pattern. The perfusion medium was a customized blood substitute. The system could keep the tissues viable and functional for more than 21 days.
The scientists could follow the drug distribution across the whole system. The interrogator can robotically add or sample the medium. The system is completely programmable. Moreover, an inbuilt microscope allows each tissue to be examined for viability and health. The researchers found that
The experiments
The first experiment was designed to test drug effects on 2 different networks of 3 organ chips (gut- liver-kidney in one, and liver-kidney-bone marrow in the second) linked by a fluidic network. This system also had a central reservoir where the ‘arterial’ and ‘venous’ blood was mixed. This helped to recreate the physiological situation where substances, including drugs, are exchanged in the blood, between various organs. It was also a convenient way to draw blood from sampling as if from a peripheral vein in the real body.
The computational results
The second paper describes a new computational scaling technique to derive dynamic changes in drug levels over time, and the adverse effects on various organs. The computation shows what would happen if the organ was of the size that is typical of the average human body. This technique also adjusts for physical adsorption of the drug into materials in the experimental system.
With the first system, the researchers added nicotine to the intestine chip, to simulate oral nicotine, often used as a gum to help smokers quit, but recently also for inflammatory bowel disease and degenerative diseases of the nervous system, on an experimental basis. The drug levels at various points were measured using mass spectrometry.
Interrogator: Human Organ-on-Chips
Interrogator: Human Organ-on-Chips from Wyss Institute on Vimeo.
With the second, the researchers explored drug distribution and effects following ‘intravenous’ administration of the commonly used chemotherapy drug cisplatin. They found the same ability to accurately predict its pharmacodynamic effects, and also its clearance in the liver. This will facilitate a more detailed understanding of its pharmacokinetics.
This is the first time that it has become possible to artificially and accurately predict dynamically what will happen to drug levels in the blood, and to provide a model for pharmacokinetics. Says co-researcher Ben Maoz, “The resulting calculated maximum nicotine concentrations, the time needed for nicotine to reach the different tissue compartments, and the clearance rates in the Liver Chips in our in vitro-based in silico model mirrored closely what had been measured previously in patients.” Another researcher Anna Harland, echoes, “Our analysis recapitulates the pharmacodynamic effects of cisplatin in patients, including a decrease in numbers of different blood cell types and an increase in markers of kidney injury.”
Significance and implications
Of course, DARPA is well aware that its goals are often over the top, but its objective is to drive research to unprecedented levels of achievement. Researcher Donald Ingber says, “We were very proud to obtain major funding support from DARPA to take on this challenge, and we are now even more proud that we have successfully met their goal, which would not have been possible without the exceptional talents, interdisciplinary spirit, and monumental team effort at the Wyss Institute.”
The project involved specialists from a host of fields, including engineering, microfabrication, pharmacology, computation, industrial modeling and physiology. Ingber sums up: “"This is what we love to do at the Wyss Institute: make science fiction into science fact. And we hope our demonstration that this level of biomimicry is possible using Organ Chip technology will garner even greater interest from the pharmaceutical industry so that animal testing can be progressively reduced over time.”
Source:
Journal references:
Herland, A., Maoz, B.M., Das, D. et al. Quantitative prediction of human pharmacokinetic responses to drugs via fluidically coupled vascularized organ chips. Nat Biomed Eng (2020). https://doi.org/10.1038/s41551-019-0498-9
Novak, R., Ingram, M., Marquez, S. et al. Robotic fluidic coupling and interrogation of multiple vascularized organ chips. Nat Biomed Eng (2020). https://doi.org/10.1038/s41551-019-0497-x