The immune system is among the most vital systems involved in making healthy life possible. It has to respond robustly when the body is threatened with infection. On the other hand, it must keep itself in rein lest the toxic chemicals it directs against invading microbes overwhelm the healthy tissues and cause damage to the host.
3d illustration of an immune system T cell killing a cancer cell. Image Credit: Meletios Verras / Shutterstock
An example of the former situation is seen in patients on chemotherapy for cancer, or people with AIDS, who have weakened immunity. As a result, the inefficient immune response allows minor infections to become dangerously widespread or intense, even causing death.
The latter scenario is typified by septic shock, a hyper-reaction by the immune system to the presence of bacterial antigens. Here the scale of toxin and inflammatory chemical production by the immune system is so high that it literally destroys multiple organs and kills the patient.
How the immune system could bring about this delicately discriminating reaction was a puzzle, which the researchers in the current study set out to unravel.
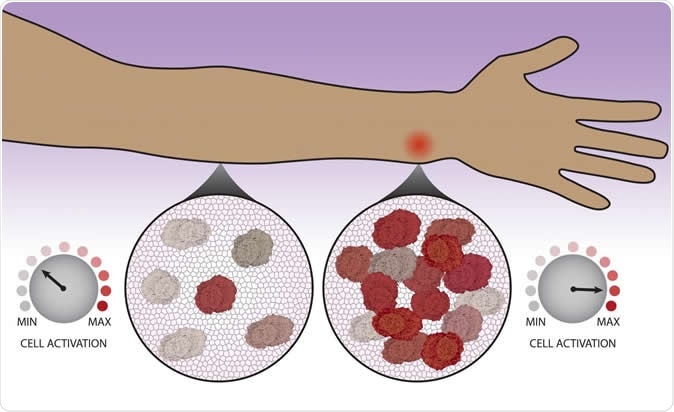
After migrating to a site to possible infection, immune cells count each other in order to decide whether to kick into high gear. Image Credit: Northwestern University / Shutterstock
Inflammation and immune activation
Immune activation is an established phenomenon, in which immune cells migrate to the site of infection or trauma and release various signaling chemicals to bring neighboring tissues and other immune cells on board. This manifests as swelling, redness, and pain, the classical signs of inflammation.
The new study recognizes that the recruitment of other cells is not an automatic or unplanned response. Still, each cell consults its neighbors as to whether more cells are required to defend the host, before activating. One aspect of this is reporting their presence to each other so as to arrive at a 'cell count', so to speak.
Researcher Joshua Leonard says, "The cells make a coordinated decision. They don't uniformly activate but instead collectively decide how many cells will activate so that together, the system can fend off a threat without dangerously overreacting."
This finding was made possible by the use of advanced computational models to interpret the experiments and thus produce a concrete idea of how cells go about deciding whether to activate themselves and therefore recruit new cells.
The study
The researchers examined macrophages, a type of immune cell that eats bacteria and other foreign particles. It is a first-line defense against infectious agents, with other innate immunity cells. The scientists watched how these cells reacted when faced with a bacterial antigen, which is one of the immune activation triggers for the host organism. The specialized techniques they used helped them observe each cell individually over a period of time.
These responses were fed into the computational model, which spat out the meaning of each of their recorded observations and helped them explain what they were seeing. The researchers found that as time passed, the cells were getting a feel of their surroundings, especially how many of each type of other immune cells are in the vicinity.
The immune cells were individually interpreting the collective presence of immune cells at the site of a threat to decide whether, on an individual basis, each of them needed to activate or not. In short, an additional layer of command is present to regulate immune response on the field.
Researcher Joseph Muldoon describes the "decentralized" functioning of the immune system; thus: "Immune cells are individual agents that need to work together. Over time, the cells observe their surroundings to get a sense of their neighbors. Each cell becomes poised to respond as a high activator or not. Cells arrive at different activation states, but in such a way that, on the whole, the population response is calibrated."
The presence of this command layer has, in Muldoon's words, opened up "a whole avenue to study whether there are new targets for immunomodulation." If scientists understand more about the way the immune system is regulated, they could design therapies to activate the immune system specifically against a tumor, sparing the rest of the body, or to reduce immune hyper-function in autoimmune diseases like lupus.
Leonard comments: "Biology has evolved so many fascinating and surprising ways to control complex processes. As synthetic biologists, we work to engineer cells to perform customized therapeutic functions. Understanding nature's innovations help us to come up with new designs and enables us to be better engineers."
Journal reference:
Muldoon, J.J., Chuang, Y., Bagheri, N. et al. Macrophages employ quorum licensing to regulate collective activation. Nat Commun 11, 878 (2020). https://doi.org/10.1038/s41467-020-14547-y