Premature baby. Image Credit: Kristina Bessolova / Shutterstock
Even as medical science advances and technology comes up with new ways to assist human life, preterm birth remains the Number 1 reason why babies die in their first year of life. For instance, 2018 marked the fourth consecutive year when the rate of preterm birth increased. So far, it had moved up from 9.6% in 2015 to 10.2% in 2018. This is since statistics began.
Bacterial pneumonia is still among the dreaded complications of prematurity, and over 1 million infants with bacterial pneumonia die each year.
Type 3 ILCs and the gut-lung axis
Babies born prematurely are at risk of not being able to breathe independently because of the incomplete development of their lungs. The lack of type 3 ILCs also predisposes them to develop bacterial infections of the lungs, including bacterial pneumonia. Those who eventually make it past this stage may end up with permanently damaged lungs, being prone to respiratory infections their whole lives, and the associated illnesses.
The study builds on earlier research showing that the presence of beneficial gut bacteria, the processes which lead to the maturation of the lungs, and the growth of innate immune cells are all interlinked to form a "gut-lung axis." This axis forms very early in newborn life, which is, therefore, a critical period of development of the lung.
The researchers have already published data showing the vital role played by the gut-lung axis in human development and how giving too many antibiotics too early could disrupt this crucial process in premature babies. In 2017, they showed how the interactions between the commensals living in the host intestine shaped the range of responses possible to the immune cells in other body sites, including the lungs.
The initial stimulus for this process is the presence of gut commensals, which stimulate dendritic cells within the intestine. Colonization of the gut with commensal bacteria occurs at birth and moves through a predefined succession of species to reach a stable equilibrium during the very first month after birth. They thus have a vital role in programming immune cells to meet bacterial challenges.
The dendritic cells are immune cells that are good at presenting antigens, or cell-related molecules, on their cell surface. This is done to alert other immune cells to the presence of antigens. In the early period of human development, the presentation of these antigens is essential to teach the body which antigens are harmless or friendly, and which are not.
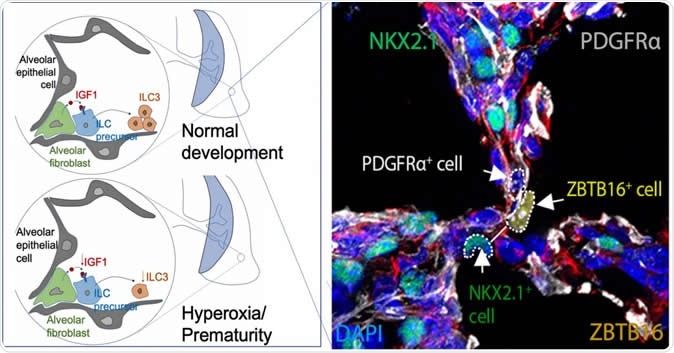
This image from a study in the journal Immunity shows fluoresced cells in the developing perinatal lung of a baby mouse as it forms air sacs called alveolar. The areas highlighted by dotted circles show the molecular components that form Type 3 innate lymphoid cells, which help the lungs develop a durable immune defense against microbial infections. Not shown is a companion comparison image that shows dysfunctional development in the lung of a premature baby mouse. The illustrations at left diagram normal and premature development of air sacs. Image Credit: Cincinnati Children's
New light on the role of IGF1
Dendritic cells cause the type 3 ILCs to produce a molecule called CCR4, which is a lung homing signal, stimulating them to migrate to the developing lung. This can occur only if the intestine is exposed to commensals during the right time, immediately after the baby is born. If this happens, normally, the lung witnesses a sudden inflow of type 3 ILCs.
The current study shows that chemicals produced by these cells prod the fibroblasts within the developing alveoli to produce a hormone called insulin-like growth factor 1 (IGF1). Higher IGF1 levels then lead to the proliferation of early-type 3 ILCs in the lungs, forming a larger population, which then matures to become functional.
The deletion of the gene that causes IGF1 in the lungs of these baby mice halted the normal development of these cells and reduced their resistance to infections of the lung and to pneumonia. In addition to examining lung tissue from premature mouse infants, the researchers also had access to the lung tissue donated by the families of human babies born prematurely.
Implications
The clinical importance of this is obvious. The commensal gut bacteria that are crucial to the development of the gut-lung axis cannot grow in the high-oxygen ventilator environment that is required by the premature babies at first to provide their bodies with enough oxygen to function, given their inadequate lung function. When they are also given antibiotics, gut bacteria are further reduced. This disrupts the normal migration of type 3 ILCs into the lung and increases the chances of pneumonia in the newborn. However, when commensals were transferred into the infant's guts immediately after birth, this susceptibility was reversed.
The study could thus lead to the development of new methods to increase innate lung immunity in preterm babies, avoiding lifelong respiratory problems. The next step is to test the theory in a laboratory mouse model to see if these new biogenetic techniques will work in preterm infant mice to help produce type 3 innate lymphoid cells in the lungs.
The preclinical study planned in laboratory mouse infants will test the usefulness of giving beneficial gut bacteria and pulmonary IGF1 to the mouse babies born prematurely, in the context of developing a stronger immune response. Both of these approaches are currently being tested in humans for other indications, so they are not new to medical science. This ought to increase the chances of the mouse building a robust and long-lasting immunity against lung infection and thus preventing inflammation of the tissues.
Journal reference:
Insulin-like Growth Factor 1 Supports a Pulmonary Niche that Promotes Type 3 Innate Lymphoid Cell Development in Newborn Lungs Oherle, Katherine et al. Immunity, Volume 52, Issue 2, 275 - 294.e9, https://www.cell.com/immunity/fulltext/S1074-7613(20)30032-7