Preclinical trials indicate that a drug candidate called OxaliTex can stop the growth of cancer cells completely. OxaliTex appears to be more potent than many other common chemotherapy drugs, with less toxicity and less rapidly counteracted by mechanisms of drug resistance. The study was reported in the journal Proceedings of the National Academy of Sciences in March 2020.
OxaliTex comprises two parts: one which has a star-shaped molecule called texaphyrin, and a modified platinum-based anticancer drug. The first molecule is the delivery system, while the second is the toxic molecule itself.
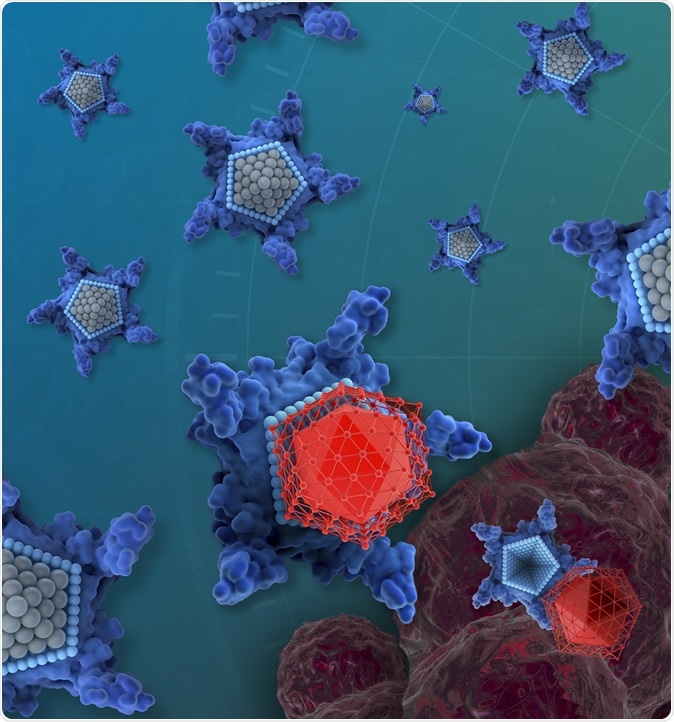
The drug candidate, called OxaliTEX, is made of two parts: a star-shaped molecule (blue) called texaphyrin that acts like a kind of delivery truck and a modified version of a platinum drug (red) that acts as a toxic package for cancer cells. Image Credit: iQ Group Global
Earlier studies in 2012 showed that the compound could overcome cisplatin resistance in cancer cells by accumulating in the cell, forming DNA adducts, stabilizing the tumor suppressor gene TP53 or tumor protein which codes for a protein that regulates the cell cycle, so as to suppress tumor formation, activating TP53, and inducing apoptosis. The last two actions were common to both sensitive and resistant cells. The drug thus reactivates the mutated TP53 gene, which is among the main reasons for resistance. This drug is, therefore, capable of overcoming two dominant mechanisms of tumor cell resistance.
There are several platinum-based chemotherapy drugs like cisplatin, carboplatin, and oxaliplatin. These have platinum incorporated into their chemical structure. The platinum forms extremely reactive complexes that bind to DNA and forms crosslinks within the molecule. Since DNA encodes the entire cellular activity of all cells, including cancer cells, lethal damage to this molecule arrests further cell growth, and induces cell death.
Platinum-based drugs are used mainly for the treatment of ovarian cancer, breast cancer, cancers of the head and neck, testicular, bladder, kidney, prostate, and many other cancers. It is estimated that this class of chemotherapeutic agents is used in the treatment of almost one of every two cancers, typically in combination with another drug category called taxanes. Taxanes also disrupt cell division by another mechanism of action.
The problem
Platinum-based drugs are known to cause kidney damage (for cisplatin), bone marrow suppression (for carboplatin), and nerve damage (oxaliplatin). Other adverse effects include anaphylactic reactions, liver damage, cardiac injury, epithelial damage to the lining of the intestine and the mouth, and weight loss. This may mean that the dose must be reduced by 25% to 100%.
Moreover, cancer cells often work around these by using inbuilt mechanisms of resistance, such as alternative pathways of metabolism. For instance, they may 'hide' the tumor antigens, inactivate the drug, alter the tumor cell molecules targeted by the drug, inhibit cell death pathways, or cause epigenetic modifications that hinder cytotoxicity.
The solution
The first difficulty is met by the increased affinity of the texaphyrin molecule for cancerous cells compared to healthy cells, which means the side effects are reduced. Secondly, the platinum drug is modified to reduce its toxicity while simultaneously making it more challenging to work around, and thus preventing the chances of resistance.
In the current study, the researchers tested the candidate drug for its efficacy, head to head against carboplatin, an approved platinum drug used to treat cancer of the ovary. The tests, carried out on tumor-bearing mice, showed that OxaliTex was much more effective than carboplatin in reducing tumor size in this group.
The tumors remained unchanged in the latter group. On the other hand, all the tumors in the mice treated with the former drug showed a complete arrest of growth until 2-3 weeks after the treatment ended. At this point, they started to grow again. The challenge now is to optimize the dose to achieve complete elimination arrive at an optimized dose, at which point they hope they can eliminate the tumors completely.
When the adverse effects were compared, it was found that the new drug OxaliTex had significantly lower toxicity than oxaliplatin, another drug approved by the Food and Drug Administration (FDA), which is used in the treatment of colorectal cancer, among other tumors.
Says researcher Jonathan Arambula, "We created something that's better tolerated than currently approved drugs." The next step is to explore the toxic properties of this candidate drug in much greater depth. Once this is complete, they hope to begin a Phase 1 clinical trial in humans within the next two years.
Journal reference:
Oxaliplatin Pt(IV) prodrugs conjugated to gadolinium-texaphyrin as potential antitumor agents Grégory Thiabaud, Guangan He, Sajal Sen, Kathryn A. Shelton, Wallace B. Baze, Luke Segura, Julie Alaniz, Ruben Munoz Macias, Greg Lyness, Alan B. Watts, Hyun Min Kim, Hyunseung Lee, Mi Young Cho, Kwan Soo Hong, Rick Finch, Zahid H. Siddik, Jonathan F. Arambula, Jonathan L. Sessler Proceedings of the National Academy of Sciences Mar 2020, 201914911; DOI: 10.1073/pnas.1914911117