Radiotherapy is a method of treating cancer using radiation. In principle, radiotherapy could use all types of radiation but the most common types are X-rays and electron beams. Less well-known and less commonly used is high energy proton beam therapy, which is only just entering the UK healthcare market.
Proton beam therapy uses a different type of particle compared to other, better-known, radiotherapy treatments but works in a similar way. The particles are used to damage cancer cells, and in the case of proton beam therapy, damage is done specifically to DNA of cancer cells. By stopping the cancer cells from reproducing, the treatment is ultimately able to destroy the cancer.
What advantage does proton beam therapy hold over x-ray and electron beam therapy?
X-ray radiation penetrates your entire body, meaning it enters at skin level and then goes all the way through your body which, as an example, allows an image to be generated in the process. Whilst X-rays are able to create good quality images, the quantities of energy deposited in the body of a patient are not ideal for treatment purposes because a lot of the energy of the beam is deposited at the entry location on the patient’s body. As the beam travels through the body of the patient, it deposits less and less energy. This means that if you have a deep-seated tumour, such as a brain tumour, then by the time you reach the tumour, you will have deposited a lot of unnecessary energy in the body of the patient.
With electron beams, the effects are even more limiting because they only have a penetration depth in water (a material that closely resembles the human body) of around 12 centimetres. Anything that is deeper than 12 centimetres can hence not be reached by electron beams. Furthermore, you still deposit most of the energy at the entry location.
Protons are something of a magic bullet because they enter your body without affecting any of the healthy tissue. They also only deposit energy where you want it to be, and are only dependent on the energy of the treatment beam. For instance, if you tune the beam to the right energy, you can tell the treatment beam to stop exactly after 13.4 centimetres in the body, after which you release that energy, to destroy the tumour only where is necessary. That's a huge difference.
When treating brain tumours, doctors are acutely aware that the brain is a very sensitive organ and any damage you do to surrounding healthy tissue could have very significant and serious side effects which could damage your sensors, or affect your ability to speak or move. However, proton beam therapy provides a treatment opportunity that destroys cancerous cells only at the site of the tumour and leaves the rest of the tissue intact. That's why proton beam therapy is a game-changing treatment for some cancer types.
Where is proton beam treatment available in the UK?
Previously in the UK, proton beam therapy was only available at very low energies, meaning that the only application was for eye tumours. This type of treatment was carried out for decades at the Clatterbridge Cancer Centre on the Wirral but since November 2019, higher energy proton beams have become available for treatment purposes since the Christie Hospital in Manchester opened its doors for proton beam therapy. There are many more centres that will soon be opening in the UK, including one in Liverpool.
How is the treatment beam tuned?
To tune the treatment beam you need a machine that provides you with a beam of protons at variable energy and intensity levels, and it needs to be able to deliver that in a very flexible way. Taking the example of the brain tumour once more, if you have a tumour that is a few centimetres in size in three dimensions located within the head of a patient, and you know the shape of the tumour very precisely, then you need to “paint” that tumour with your proton beam in order to fully treat it.
As a result, you need a beam delivery system that allows you to not only scan transversely but also longitudinally, through that volume and deposit exactly the right energy to destroy the cancer at every point inside the volume of the tumour. Your facility needs to be able to accelerate a beam of protons to the required energy and then deliver it precisely to the volume within the patient where it is required. This is all done with a particle accelerator.
Looking towards the future, how can medical accelerator research help to improve radiotherapy for patients?
One of the main advantages of proton beam therapy is that it essentially has no immediate side effects. When you look at alternative forms of treatment, many of them can be quite traumatizing for patients, especially chemotherapy which can damage their entire body and has very significant effects on their wellbeing. The side effects can be extreme, preventing patients from leading a normal life during treatment periods.
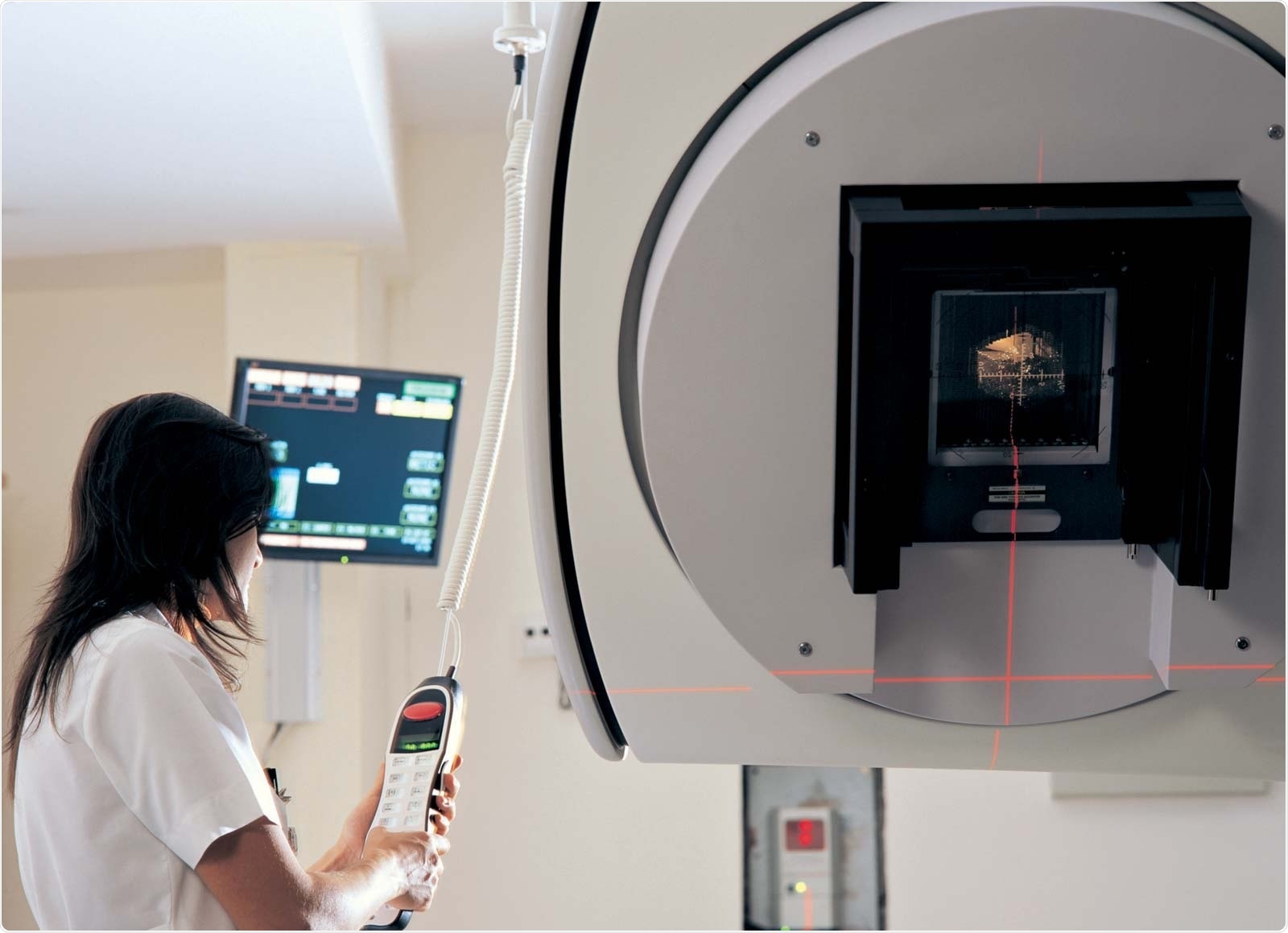
Image credit: Optimization of Medical Accelerators Project
With proton beam therapy, when the patient goes into the centre they receive their treatment on the day, and treatment is typically spread over 10 to 20 treatment days. On the day they go into the centre, they have some preparation in the treatment room, they lay down on the treatment bench and then they receive their radiation, which they don't feel at all. It has no immediate side effects. There are no other major drugs patients have to take after that point, but after 20 treatments the tumour can be destroyed.
It's clearly not the magic bullet for all cancers, but for very highly located tumours, such as brain tumours or prostate cancer, it really is a game-changing treatment.
Can you provide an overview of the EU-funded Optimization of Medical Accelerators (OMA) research project that you led?
For the OMA project, our focus from the beginning was to carry out cutting edge research in proton and ion beam therapy. We decided to address this from a clinical perspective, from an engineering point of view when it comes to the design and construction of these machines, and also from a physics and biology side considering the understanding of how these beams actually interact with cells in the human body. We wanted to model these processes better and help improve understanding.
With OMA, we pulled a lot of the expertise that is available across Europe together and built bridges between clinical centres, research facilities where this treatment was pioneered, leading universities who produce innovative ideas, and companies that are specialised in providing these treatment facilities. In collaboration, we aimed to significantly improve treatment.
From a clinical perspective, one of the important challenges to tackle is the uncertainty around energy deposition inside a patient. A clinician would really like to know where exactly the beam delivers its energy. At the moment, that's one of the main issues that prohibits even more precise and targeted treatment. Significantly reduced safety margins and, therefore, reduced side effects, could be gained by more accurate prediction and verification of this range in the body of the patient.
The first direct measurement device for clinical use for that purpose is a “prompt-gamma camera”. This was one of the technologies that was investigated and improved by one of our OMA Fellows. We had other Fellows who worked together with them, who then tailored treatment plans that take into account improved detectability.
We also developed detectors that were used as fast-range detectors, for mixed beams of carbon ions and helium ions, so that we could online monitor the range of the beam and the patient. We also developed novel designs for beam delivery systems that would allow the beam to be even more precisely delivered to the patient.
We had other Fellows within the OMA project who focused more on diagnostics and they developed new detector types that enable more precise and online ways of measuring the beam as it travels to the patient, as well as diagnostics that measure the beam whilst it is inside the patient. Our diagnostics work package has really improved the overall way in which we can monitor the beam at all times, both, in the machine, as well as in the patient.
We have developed a very significant amount of software tools, which now allow both physicists that are looking into the beam transport and medical doctors that look into modelling the dose distribution inside the patient to gain far better capabilities in predicting what's going to happen when the machine is operated.
How do Innovative Training Networks work and what are the benefits of the scheme?
The traditional way in which we train postgraduate students is through a series of lectures, seminar talks and, primarily, by working on their research projects. Fundamentally, Innovative Training Networks (ITNs) believe that the best way of training these postgraduate students is by collaborating with the best partners across Europe and taking a cohort approach. ITNs train around 15 very highly qualified Research Fellows at a time and, in the case of OMA, they all work in the same broad research area but with distinct, individual projects.
Some researchers are based in industry, some of them are based at universities and others are based at clinical centres. They meet on a regular basis, not only to exchange their research progress but also to discuss how their training has been going and to discuss future collaboration. We want to get new research results from them, but at the same time, we want to train them to become world leading experts in their field.
The ITN scheme provides a unique and fantastic framework to do this, because it covers the salaries of these 15 Fellows. It also provides us with funding to organise international schools and workshops where we bring them together on a very regular basis, allowing for knowledge exchange where we have now developed all of them to a level that they can graduate to PhDs.
The OMA network was funded by the EU through the Horizon 2020 Programme. The project received €4 million of funding, which was awarded on a competitive basis. Every year around 1,500 proposals are submitted for this scheme, making it one of the most competitive in Europe and it has been for many years. A small fraction, around 5% of these proposals, are funded, so with OMA we were very proud to receive this outstanding support.
OMA was actually the very first and, so far, the only ITN proposal that received a 100% evaluation mark across any scientific discipline. To put that into perspective, that's out of around 15,000 proposals, and our project was the only one that received a 100% mark. To me, that was a clear indication that this is a very important area for science, technology and clinical practice to improve healthcare all across Europe.
How important is it to invest in fundamental scientific research? What are the benefits for society?
This is a very interesting question, particularly when considering OMA. I would dare to say that pretty much all the accelerator technology in OMA would not exist had there not been fundamental research conducted decades earlier for particle and nuclear physics applications.
My view is that there needs to be a balance between fundamental science and applied research that addresses the open questions that we have right now. However, the significant breakthroughs, these ground-breaking discoveries that lead to technology changes that ultimately change society, will only ever happen if you invest in fundamental research because that's what pushes the boundaries. Fundamental science is not something that tries to find an answer to a societal problem we have now, it tries to look well beyond that. The technologies that arise from fundamental science ultimately drive innovation.
What is the future for your research and for medical accelerator R&D as a whole?
Accelerators form the backbone of current research in all scientific disciplines. If I look at strategic roadmaps like the European Strategic Facilities Roadmap, then around 50% of future science infrastructures rely on accelerator technology, one way or another.
This presents a number of challenges. We want to reach ever higher energies to look deeper into the nature of matter. We want to see if we can potentially find new particles and new symmetries. One of the challenges is how we can do this, and how we can go to even higher energies.
At the same time, we want to reduce the costs, the complexity and the footprint of these facilities. We are looking at developing technologies that can make particle accelerators much smaller. There are different approaches that we are all involved in at Liverpool. We are targeting a reduction in cost and size by a factor of a 100 or a 1,000. Over a timeframe of 10 years, we are optimistic that this can be achieved for some applications.
Coming back to medical applications, I mentioned that the infrastructure at the Christie was opened last year. That was a very significant investment. It is a huge building containing a complex accelerator. If we now look 10 years into the future and we could reduce the footprint of that facility by a factor of 100 and also reduce the total costs, then the question would no longer be, "Can we have one or two of these facilities in the UK?" The question would then be, "Can we have one or two of these facilities in every hospital in the UK?" Then, of course, the healthcare benefits for patients would be massive.
How has the OMA project improved collaboration in the cancer research field?
In January 2020, we had the final project review for OMA with our Steering Committee and it was a fantastic experience. We've seen our 15 Fellows grow from excellent undergraduate students with little background in the area of medical applications, to become leading experts in their field over a period of just three years. Our Fellows are now invited to give talks at the world's most important medical conferences, and we've seen how the OMA network has created lasting links that will long outlive the duration of the funded-project.
As a result, we decided to continue pretty much all of our activities for at least two more years. In Liverpool, we are now bidding for additional funding for research projects that are connected to medical accelerators, often with other OMA partners. To some extent, the OMA project is still only at the beginning. There's clearly much more to do in proton beam therapy research and seeing now the benefits, both in terms of research outcomes as well as to the researchers that were involved, we definitely want to continue that journey.
Where can our readers learn more?
Readers can learn more about the OMA project at www.oma-project.eu, where there is a lot of information about the project in a brochure that people can download or view online. The website also contains a lot of background information about all of the different projects and also the publications that resulted from it.
For information on the University of Liverpool’s Physics Department, readers can visit www.liv.ac.uk/physics.
For my own research group, information can be found at www.quasar-group.org, where we go into detail about our research and our outreach activities.
About Professor Carsten Welsch 
Professor Carsten Welsch studied physics and economics at the Universities of Frankfurt in Germany and UC Berkeley in the United States. He received his PhD in accelerator physics at the University of Frankfurt and after some years did Postdoc research at the Max Planck Institute for Nuclear Physics in Heidelberg, Germany. He was awarded a Fellowship at CERN in Switzerland. He founded the pan-European QUASAR Group in 2007.
Professor Carsten Welsch has been a member of the academic staff at the University of Liverpool and a member of the Cockcroft Institute of Accelerator Science and Technology since 2008. In 2011 he was promoted to Full Professor of Physics and has been Head of the Physics Department since September 2016.