Coronaviruses are a group of RNA viruses that cause diseases in mammals and birds. In humans and birds, they cause respiratory tract infections that can range from mild to lethal. Mild illnesses in humans include some cases of the common cold. In contrast, more lethal varieties can cause SARS, MERS, and now COVID-19 disease. The most recent type of coronavirus, severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2), along with the previous SARS-CoV-1 and MERS-CoV, fall under the category of beta coronaviruses. There have not yet been any notable developments in the treatment approach for these beta type CoV, which makes it compelling to design and develop a unique therapeutic route to minimize the transmission of SARS-CoV-2 and impact of COVID-19.
The COVID-19 disease caused due to the SARS-CoV-2 virus causes a wide range of illnesses starting from mild respiratory illness, severe progressive pneumonia, and acute respiratory distress syndrome, elevation of cytokines, multiple organ failure, and death. Neutrophils, which constitute about 70% of white blood cells in our body, are higher in the severe progression of COVID-19 disease. The abnormal activation of neutrophils triggers the release of neutrophil extracellular traps (NETs) and extracellular DNA leading to multiple organ failure and septic shock.
As the treatment of severe COVID-19 is very difficult, the researchers from the Korea Research Institute of Biosciences and Biotechnology (KRIBB) suggested that DNase-1 can be utilized to dissolve the neutrophil extracellular traps (NETs), hence halting the further progression of COVID-19. Since the DNase has a short half-life in blood plasma, Wonha Lee and colleagues proposed coating DNase on the nanoparticles' surface to stabilize and preserve the DNase activity. The researchers used Polydopamine for the attachment of DNase to the nanoparticle surface. The research is published in the journal Advanced Science.
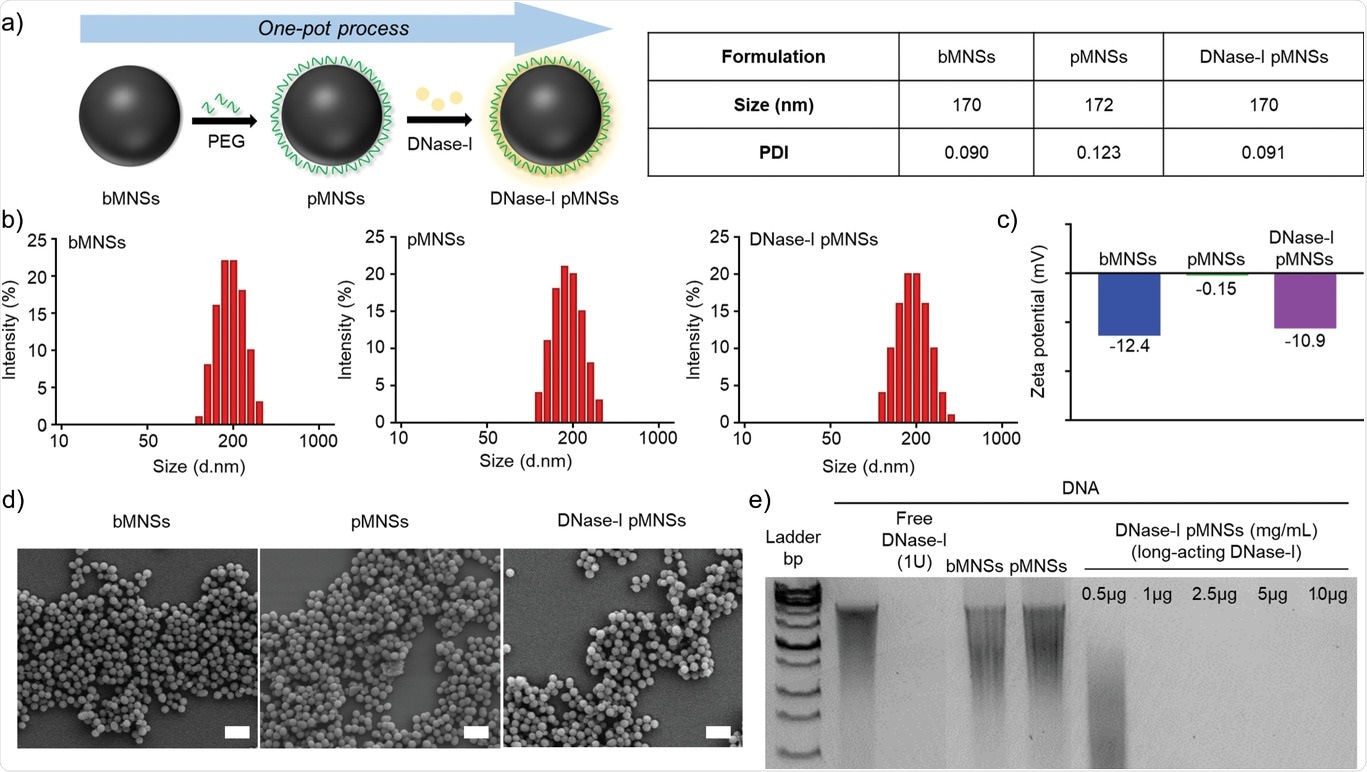
Physicochemical characterization of pMNSs. a) Preparation of DNase‐I pMNSs. b) Size distribution of bMNSs, pMNSs, and DNase‐I pMNSs. c) Zeta potentials of bMNSs, pMNSs, and DNase‐I pMNSs. d) Scanning electron microscopy (SEM) images of bMNSs, pMNSs, and DNase‐I pMNSs (scale bar: 500 nm). e) Migration profile of pure DNA after digestion with free DNase‐I, pMNSs, and bMNSs, as well as various amounts of DNase‐I pMNSs. pMNSs, PEG‐coated melanin‐like nanospheres; bMNSs, bare bioinspired melanin‐like nanospheres.
Development of the Novel Treatment Strategy for COVID-19
Initially, the researchers analyzed the Computed Tomography of severely infected COVID-19 patients whose lungs were intensely damaged. They found that the patients had higher neutrophil count and less lymphocyte count, another type of white blood cell responsible for fighting infections. They also compared the extracellular DNA, DNAse, and Citrullinated Histone H3 in healthy and COVID-19 infected patients. Lee found that the extracellular DNA and citrullinated Histone H3 levels were on the rise in severely infected individuals, whereas the DNase –I level decreased in infected patients.
Subsequently, the scientists synthesized the melanin like nanoparticles using the oxidation of dopamine. The surface of the melanin-like nanoparticles was immobilized with DNAse – I and Polyethylene Glycol (PEG). The visualization of synthesized and coated nanoparticles under Scanning Electron Microscopy revealed that the nanoparticles' morphology was spherical and had uniform size distribution. The researchers evaluated DNAse – I and PEGylated nanoparticles' degradation capability and found that the DNA was degraded at a nanosphere concentration greater than 1microgram. They also assessed the DNAse-I binding efficiency and stability over time on the nanoparticles through BCA assay and treatment with FBS with PBS and only PBS media. As the concentration of DNAse – I increased, the binding efficiency also increased, and the DNASe-I coated nanospheres were stable up to 72 hours in only PBS media.
Effect of DNAse- I PEGylated Melanin-like Nanoparticles on COVID-19 patients
Severely affected COVID-19 patients suffer from a decline in DNAse-I in their system, which necessitates the use of DNAse-I PEGylated melanin-like nanoparticles. The group of scientists from the Korea Institute of Biosciences and Biotechnology treated the plasma of COVID-19 patients and surprisingly found that both free DNAse, as well as DNAse, coated nanoparticles reduced the extracellular DNA levels. Furthermore, they found that the factors such as myeloperoxidase, neutrophil elastase responsible for neutrophil extracellular traps that consequently progress the severity of COVID-19 was also alleviated to a greater extent when treated with DNAse-I PEGylated melanin-like nanospheres. More than half of the COVID-19 patients admitted to the hospital had sepsis caused by uncontrollable inflammatory responses. The inflammation is characterized by increased levels of cytokines. They quantified the immune factors responsible for inflammation after treatment with DNase-I and DNAse-I coated nanoparticles, and they found that the factors reduced considerably, promising that these nanoparticles coated with DNAse can be used as a potential treatment approach.
Effect of nanoparticles on Cecal Ligation and Perforation (CLP) treated Septic mouse model
The researchers further validated the synthesized DNase- I PEGylated nanoparticles in vivo using a CLP mouse model. To create a CLP model, the large intestine's opening called the cecum is cut open, and holes are created to induce inflammation, mimicking sepsis in humans. The KRIBBS scientists administered DNase-I melanin=like nanospheres onto the CLP mouse model and found that the neutrophils' number reduced dramatically. The scientists also realized a 40% survival rate and a reduction in cytokine levels in the CLP models upon administration of nanoparticles, confirming that these nanoparticles can be used as a potential treatment strategy.
Conclusion
The researchers demonstrated that extracellular DNA, one of the factors responsible for the progression of COVID-19, can act as a probable target for the treatment of sepsis-induced by the SARS-CoV-2 virus. They also found that the delivery of DNase-I PEGylated melanin-like nanospheres suppressed the extracellular DNA expression, which in turn slows the advancement of sepsis in severe COVID-19 patients. They say that early injection of these nanoparticles can potentially curtail the progression of the COVID-19 disease. Although bioinspired nanospheres' efficiency in both in vitro testing of blood samples and in vivo testing of the septic mouse model is verified, Lee recommends validation of the nanoparticles under appropriate in vivo sepsis-induced animal models before proceeding to clinical trials in human COVID-19 patients. That said, the PEGylated melanin-like nanospheres could be a promising treatment approach for SARS-CoV-2 sepsis.