A Bayesian analysis of the RECOVERY trial suggests the immunosuppressive drug tocilizumab can reduce deaths in hospitalized COVID-19 patients taking corticosteroids as well as patients on oxygen or non-invasive ventilation and likely increases hospital discharge rates. However, it is not clear if patients on invasive ventilation also benefit.
The trial was designed and analyzed using the null hypothesis significance testing. But, this analysis method has limitations in subgroup analysis, and the consistent results obtained across all subgroups can be refined further to provide a better understanding and differences among the subgroups.
An alternative is to use a Bayesian approach. This method can include results from other randomized controlled trials and can directly address the question of how effective tocilizumab is in different patient subgroups.
Researchers from Brazil, the USA, and Canada reanalyzed the tocilizumab data using Bayesian methods to obtain better insights into its effect on different patient subgroups. They published their results on the medRxiv* preprint server.
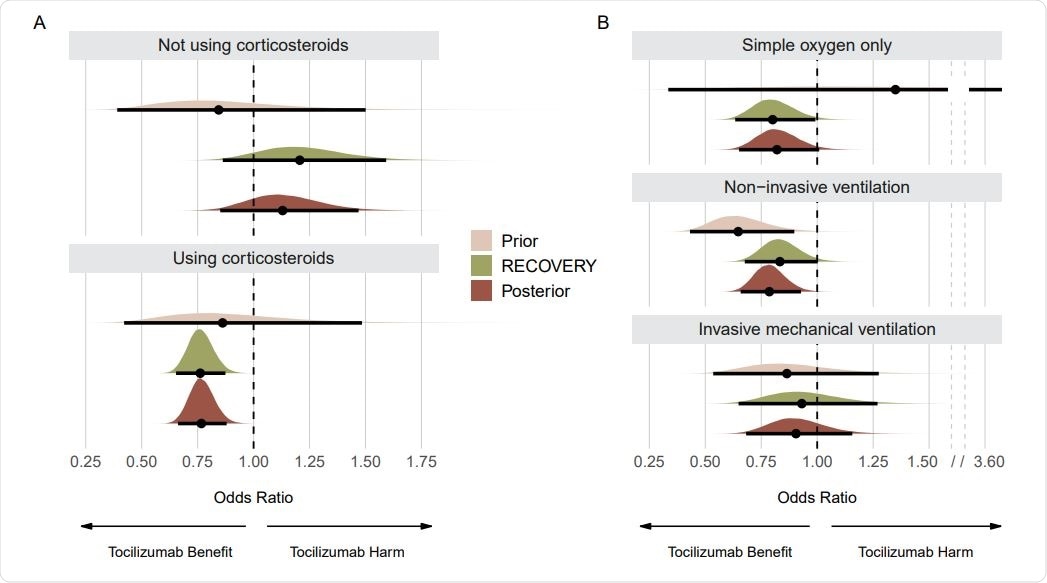
Prior, RECOVERY, and posterior distributions for each subgroup on the mortality outcome. Panel A shows results for subgroups regarding use of corticosteroids. Panel B shows results for subgroups regarding respiratory support. Point estimates depict the median and interval bars depict 95% highest density intervals. Using conjugate normal analyses, these distributions were originally combined in the log-odds ratio scale (Methods section). They were transformed into the odds ratio scale for this figure to aid visual interpretation.

 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
Analyzing benefits
The RECOVERY trial was conducted on 4,116 adults hospitalized for COVID-19 in the United Kingdom, exploring if the drug could reduce deaths and hospital stays. The team reanalyzed deaths and discharge from hospital within 28 days in two specific subgroups: patients receiving or not receiving corticosteroids and the effect of tocilizumab as a function of respiratory disease severity.
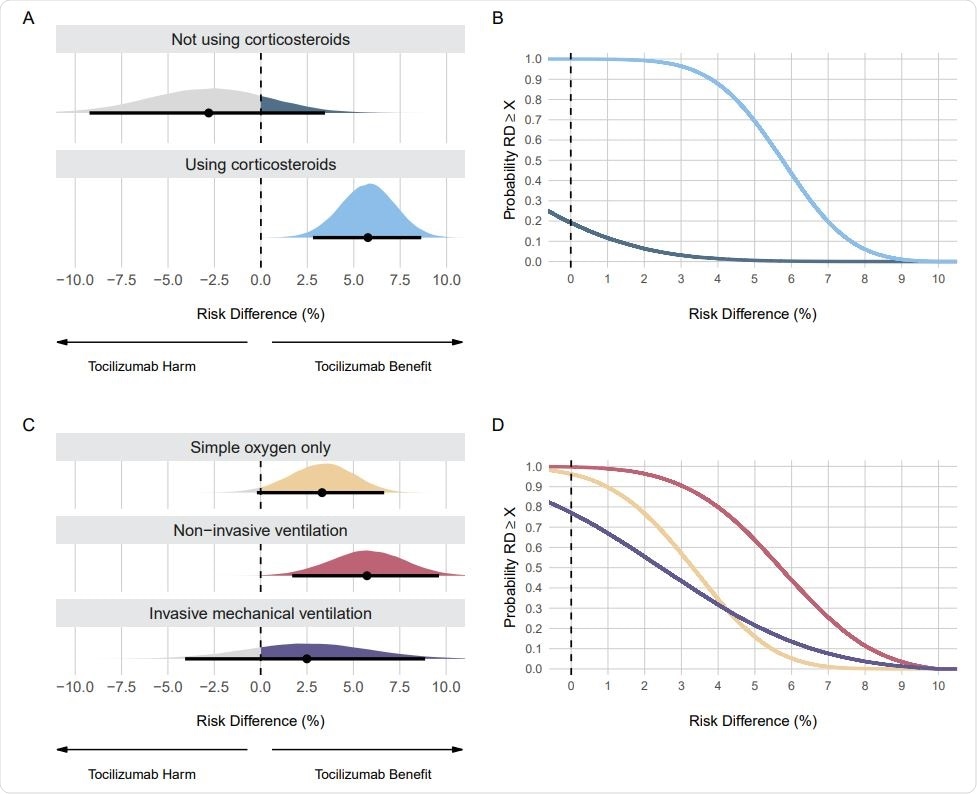
Posterior distributions and probabilities using evidence-based priors on the mortality outcome. Panel A shows the posterior distributions and Panel B shows the cumulative posterior probabilities on subgroups regarding the use of corticosteroids. Panel C shows the posterior distributions and Panel D shows the cumulative posterior probabilities on subgroups regarding respiratory support. Panels A and C: Point estimates depict the median and interval bars depict the 95% highest density intervals. Panels B and D: Cumulative posterior distributions correspond to the probabilities that the risk difference (RD) is lower than or equal to the effect size on the X-axis. The colors in Panels B and D match the ones used in Panels A and C
One component of the Bayesian analysis is choosing evidence-based priors, and there are many ways of doing that. Here, the authors used data from other tocilizumab COVID-19 randomized control trials to create priors based on evidence for each subgroup.
The analysis showed tocilizumab is especially beneficial for patients on corticosteroids and those not requiring ventilation. When looking at mortality, in patients not using corticosteroids, the probability of a risk difference benefit was about 19%. In patients using corticosteroids, a risk difference benefit probability was more than 99%, a striking difference. There is about a 96% probability that tocilizumab saved at least three lives in patients receiving corticosteroids.
Patients on non-invasive ventilation showed the most significant benefit from tocilizumab. There was also a benefit for patients receiving only oxygen. The least certainty of any benefit from tocilizumab was seen in patients receiving invasive mechanical ventilation, with a 77% probability of any benefit, highlighting that there is insufficient evidence of any benefit of tocilizumab in this subgroup.
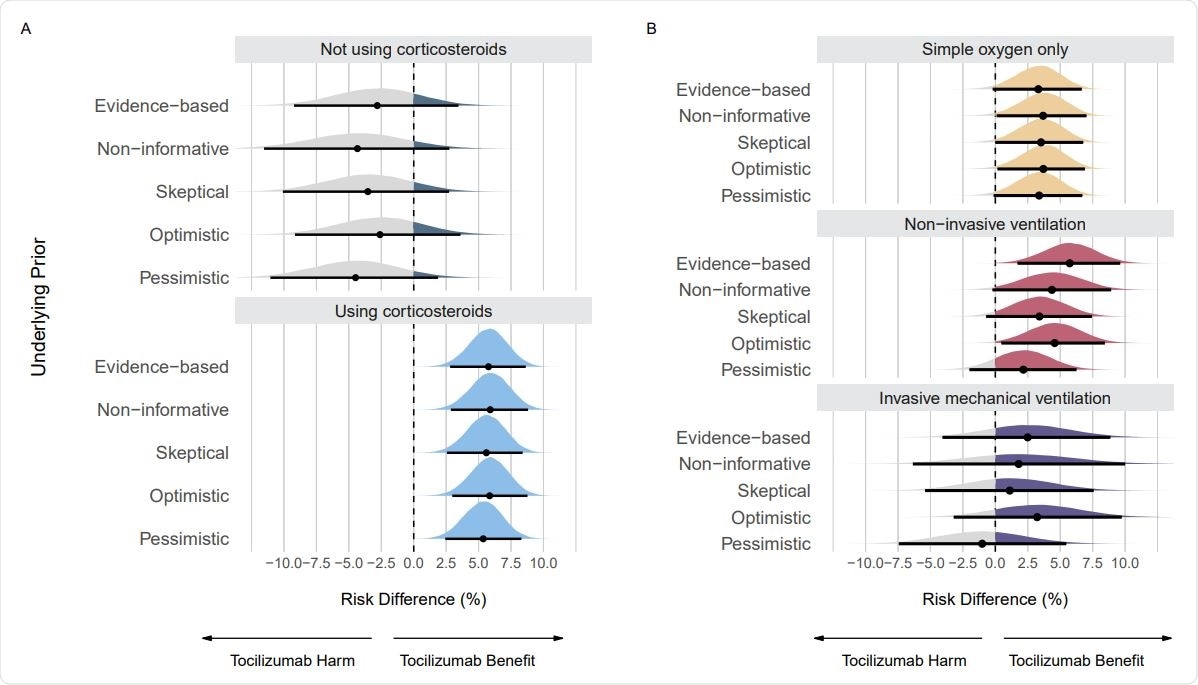
Posterior distributions from sensitivity analyses using different priors on the mortality outcome. Panel A shows posterior distributions on subgroups regarding use of corticosteroids. Panel B shows posterior distributions on subgroups regarding respiratory support. Point estimates depict the median and interval bars depict the 95% highest density intervals.
Benefits differ based on other treatments used
In the original analysis of RECOVERY data, the authors stated that they observed similar results across all subgroups. However, the authors of this new study write, “Here, we present a more in-depth analysis and interpretation of these results, casting doubt about the claim of tocilizumab’s universal benefit across subgroups.”
The probabilities of small and significant benefits are very different between patients receiving or not receiving corticosteroids. In subgroups receiving respiratory support, the probabilities of larger effect sizes are different, in contrast to the original RECOVERY analysis. This suggests more evidence is needed for the benefit of tocilizumab in patients with invasive mechanical ventilation.
The results here are different from other studies that have analyzed the effect of tocilizumab. This is likely because of the different statistical models used here, which provide a better understanding of how tocilizumab benefits patients in different subgroups.
Other studies have also found that corticosteroids without tocilizumab can also reduce deaths, especially in patients with severe disease. Here, the authors found that tocilizumab has a more significant and more certain effect in reducing deaths in people with less severe disease. In addition, there is a low probability of tocilizumab’s benefit in patients not on corticosteroids.
Tocilizumab also increases the hospital discharge rate, according to the analysis. “Thus, tocilizumab most likely provides a relevant clinical improvement in patients at different clinical severity, except for the invasive ventilation group and the non-corticosteroid users where additional data are needed,” write the authors.

 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources