Glaucoma can cause irreversible loss of vision, with IOP elevation due to abnormal circulation in the eye’s aqueous humor. Therefore, long-term and continuous IOP tracking is necessary to identify IOP fluctuations leading to ocular damage.
Current IOP measuring devices such as tonometers require trained clinicians and may not identify critical IOP fluctuations. Existing anti-glaucoma drug delivery methods such as eye drops rely on topical routes for decreasing IOP. However, such routes have been challenging due to low bioavailability, side effects, and poor compliance. High-risk conditions such as acute angle-closure glaucoma which occur due to sudden IOP elevation (IOP >21 mmHg), usually present with nausea and headaches that hinder the self-administration of drugs by patients.
Contact lens-based wearable IOP sensors integrated with microfluidic chips, resonant circuits, and photonic and piezoresistive crystals enable wireless IOP tracking and electrical drug delivery and, therefore, can be highly beneficial for glaucoma patients.
Study: Intelligent wireless theranostic contact lens for electrical sensing and regulation of intraocular pressure
Fabrication of the WTCL sensor
The sensor utilizes the sensing modulus with the inductor-capacitor-resistor (LCR) circuit for IOP sensing and multiple delivery moduli with the wireless power transfer (WPT) circuit for drug administration.
The sensor comprises a soft lens that can conformally interface with the ocular cornea and can efficiently deform for transducing corneal limbus expansion to the LCR circuit on IOP elevation and could exert external (chemical or electrical) stimulations locally onto the cornea. The lens has a double-layer structure which yields compactness and facilitates the incorporation of multiple electric drug delivery moduli and the LCR circuit in the limited and curved space of the contact lens. The linear range of the sensor is >5 to 50 mmHg, indicative of the wide breadth of IOP monitoring.
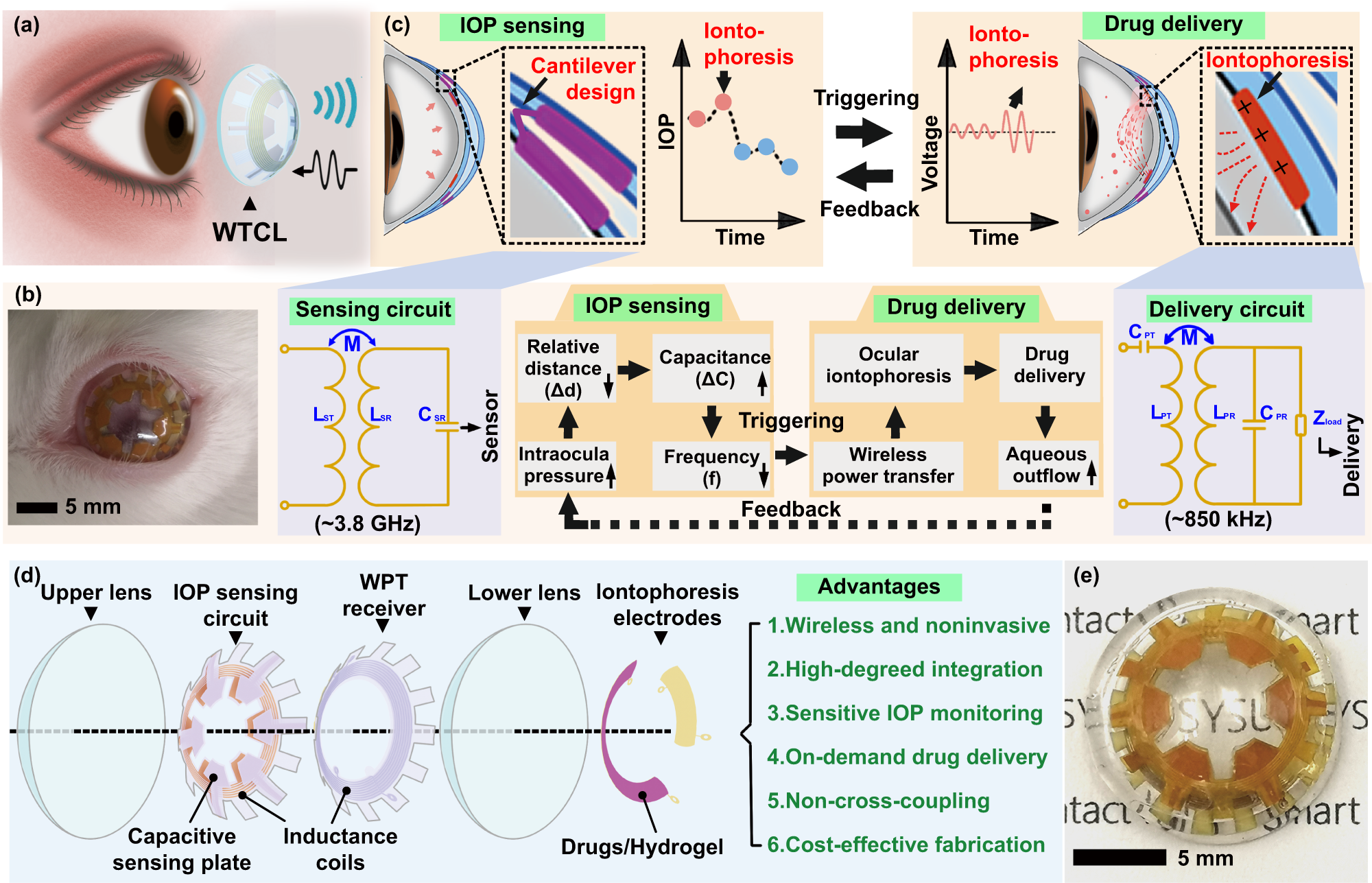 a Schematic of the WTCL for wireless IOP monitoring and administration. b Photograph of WTCL worn on the eyes of a live rabbit. c Schematic of wireless operation for the purpose of IOP monitoring and on-demand medicines administration in a minimally invasive manner. The soft device, engineered as a double-layer contact lens structure, was integrated with an LCR and a WPT receiver circuit. These modules were wirelessly connected to an external integrated antenna that could record the IOP signal and trigger iontophoresis for drug delivery if needed. Insert figures respectively highlight critical IOP sensing and drug delivery unit. d Structure of the WTCL in an exploded view. e Optical image of the WTCL.
a Schematic of the WTCL for wireless IOP monitoring and administration. b Photograph of WTCL worn on the eyes of a live rabbit. c Schematic of wireless operation for the purpose of IOP monitoring and on-demand medicines administration in a minimally invasive manner. The soft device, engineered as a double-layer contact lens structure, was integrated with an LCR and a WPT receiver circuit. These modules were wirelessly connected to an external integrated antenna that could record the IOP signal and trigger iontophoresis for drug delivery if needed. Insert figures respectively highlight critical IOP sensing and drug delivery unit. d Structure of the WTCL in an exploded view. e Optical image of the WTCL.
The dielectric air film sandwiched between the contact lens layers was combined with the LCR circuit into a cantilever configuration, forming the transducer for detecting IOP fluctuations and transmitting them wirelessly by producing signals at the resonant frequency. The ultra-softness (zero viscoelasticity and ultra-low modulus of elasticity) of the sandwiched air film facilitates ultrasensitive IOP sensing by the WTCL sensor.
The LCR circuit has a snowflake-shaped design, in which the capacitive sensing plates (a total of six plates) and the reference plates are folded to yield a cantilever structure. The design increases the sensitivity, repeatability, and reliability of the WTCL sensor.
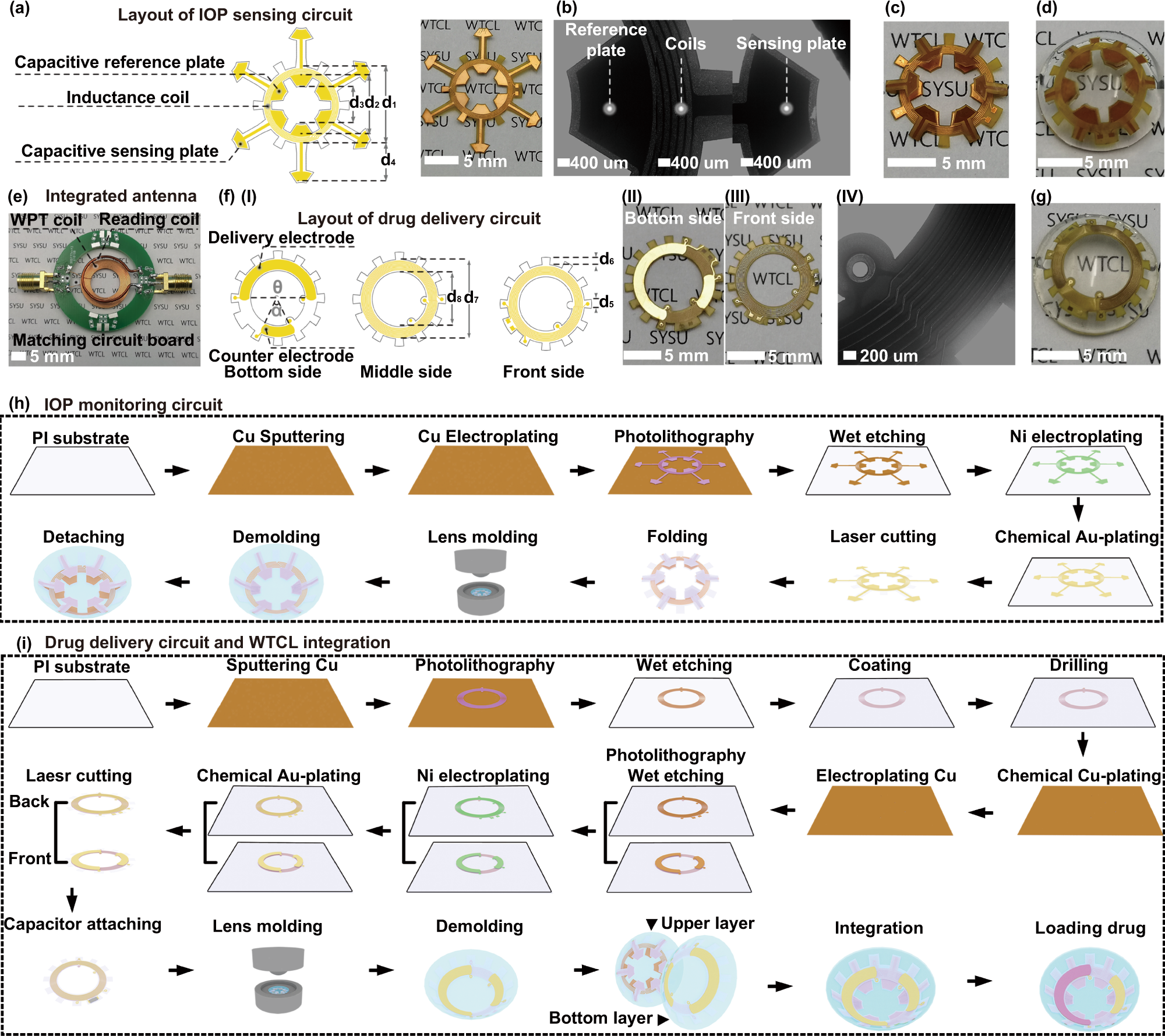 a The snowflake-shaped layout design and the photograph of the sensing circuit. b The microscopic image of the reference plate, coils, and sensing plate deployed on the sensing circuit. The photograph of (c) the folded sensing circuit and (d) the upper layer of contact lens. e Image of the integrated antenna. f (I) The flower-shaped layout design, (II) bottom surface, (III) front surface images, and (IV) microscopic image of the drug delivery circuit. g The photograph of the bottom layer lens integrated with the drug delivery circuit. Illustration of the fabrication process of (h) IOP monitoring circuit, i drug delivery circuit and the device integration. The fabrication of the sensing and delivery modulus employed a printed circuit process coupled with a cast-molding method.
a The snowflake-shaped layout design and the photograph of the sensing circuit. b The microscopic image of the reference plate, coils, and sensing plate deployed on the sensing circuit. The photograph of (c) the folded sensing circuit and (d) the upper layer of contact lens. e Image of the integrated antenna. f (I) The flower-shaped layout design, (II) bottom surface, (III) front surface images, and (IV) microscopic image of the drug delivery circuit. g The photograph of the bottom layer lens integrated with the drug delivery circuit. Illustration of the fabrication process of (h) IOP monitoring circuit, i drug delivery circuit and the device integration. The fabrication of the sensing and delivery modulus employed a printed circuit process coupled with a cast-molding method.
The corneal curvature deformation which occurs on IOP elevation compresses the air film thickness, leading to capacitance rise (CSR) and a decrease in the LCR resonant frequency which can be wirelessly recorded with the help of reading coils of the sensor’s integrated antenna.
The WPT circuit enables in situ drug administration and the migration of anti-glaucoma drugs into the aqueous chamber via iontophoresis. The WPT circuit has a flower-shaped design which enables efficient mechanical interlocking of the WPT circuit with the lower lens layer.
The sensing module comprises copper (100 µm) covered by nickel/aluminum. The delivery module comprises the same substances patterned into coils to form the WPT receiver with an additional layer of copper connected to the coils through holes. Capacitors are soldered onto the circuit for tuning the WPT frequency. The delivery electrode of the lower lens is coated with a thin layer of drugs-loaded hydrogel and assembled with the upper lens to form the WTCL sensor.
Ex vivo and in vivo performance of the WTCL sensor
The IOP sensing performance of the WTCL sensor was assessed ex vivo using porcine eyeballs. IOP was increased stepwise using saline injections, and the WTCL resonance frequency at each IOP was recorded. The results showed that an increase in IOP led to a reduction in the resonant frequency and that the WTCL sensor was a sensitive and reliable device for IOP monitoring.
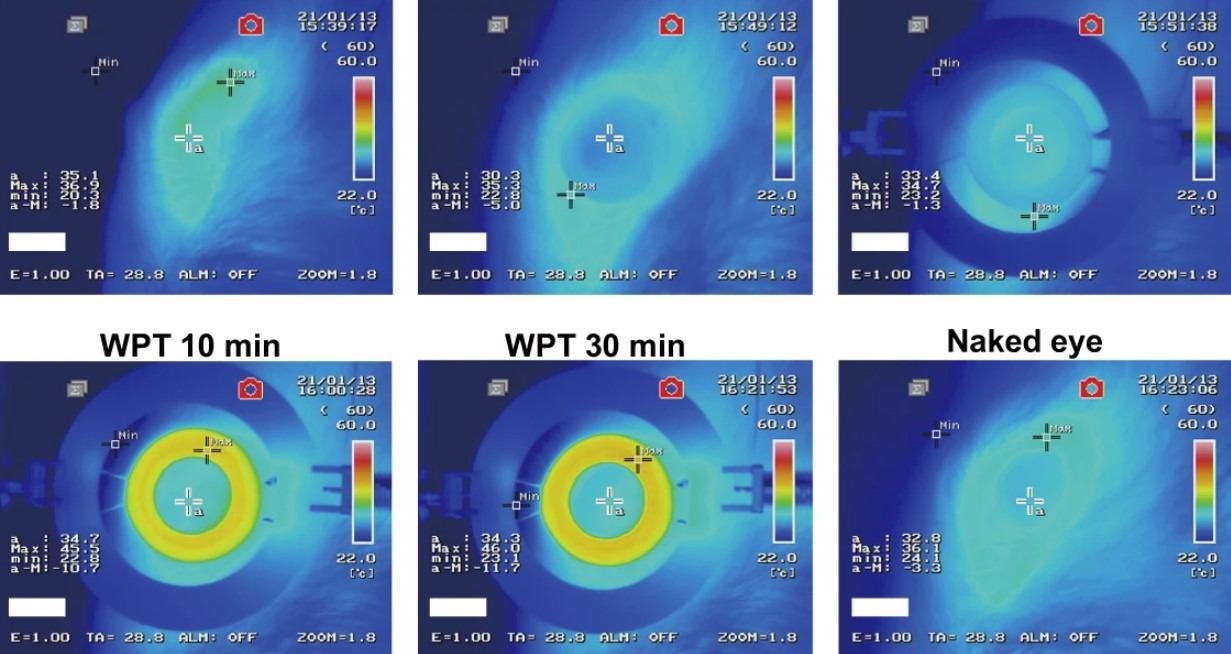
The drug-delivery performance of the WTCL sensor was tested ex vivo by drop-casting a UVB-irradiated brimonidine-loaded hydrogel layer into the porcine eyes via iontophoresis. On wireless power transmission, the WPT receiver generated alternating voltages, which led to the electric migration of the positively charged brimonidine drug into the aqueous chamber of the porcine eyes. The results showed that the WTCL sensor could be used for electrical and on-demand ocular drug delivery.
The in vivo performance of the WTCL sensor was assessed using rabbits’ eyes into which the sensor was incorporated, and the IOP measured by the sensor was compared to that by Tonopen (tonometer). In addition, brimonidine was delivered by the sensor and eyedrops and the results were compared. The results confirmed that the sensor could be used to rapidly reduce IOP with prolonged effects, which is desirable for IOP regulation.
Conclusion
Overall, the study findings showed that the WTCL sensor was a wireless, minimally invasive, sensitive, reliable, soft, compact, lightweight, and reusable device for in situ IOP monitoring and effective and on-demand ocular drug delivery. In addition, the sensor fabrication is compatible with the standard manufacturing process, highlighting its potential for large-scale use.