Dr. Cecilia Domínguez Conde: I have been a postdoctoral fellow in the Teichmann lab at the Wellcome Sanger Institute since 2019, where my focus has been to dissect the diversity of human immune cell types across lymphoid and non-lymphoid tissues. Before coming here, I did my Ph.D. in Immunology at the Research Centre for Molecular Medicine (CeMM) in Vienna. I dissected the genetic cause of molecularly undiagnosed primary immunodeficiencies using exome sequencing. In both of these research areas, the power of sequencing technologies and computational approaches has been at the core.
Immune cells can be split into various groups depending on their function and how they are activated. Could you briefly overview the different groups of immune cells and their functions, noting any previously unexplored populations?
Dr. Chenqu Suo: The immune system is a dynamic network that makes up thousands of different cell types distributed across the body. Both atlases are concentrated on immune cells in the tissues, which play a central role in the human immune system and are often understudied compared to those circulated in the blood.
All immune cells develop from the same starter cells before becoming specialized. While there are many different types of immune cells, they can be grouped into two main categories. One of these, known as myeloid cells, includes macrophages, monocytes, and dendritic cells. These cells can quickly detect infections. When they encounter a pathogen, they send signals to the rest of the body to start mounting a quick immune response.
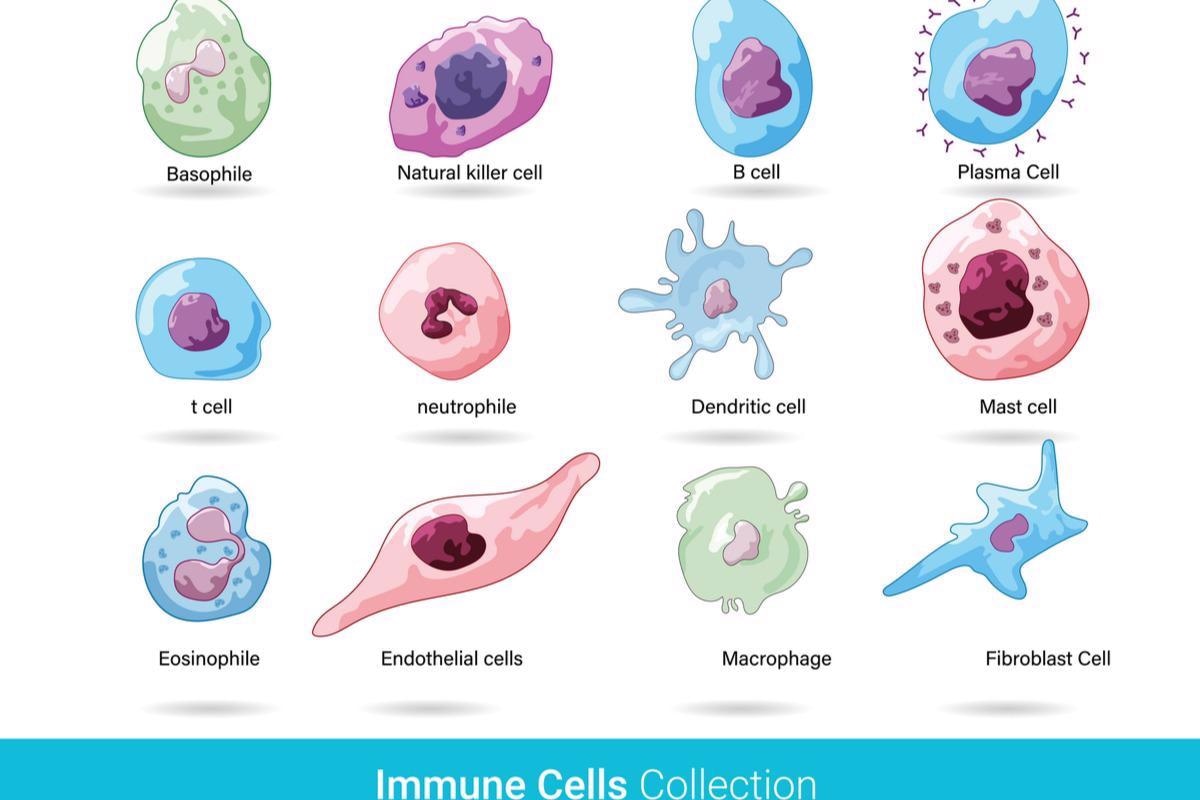
Image Credit: Art of Science/Shutterstock.com
The other group, lymphoid cells, includes B cells and T cells that preserve the memory of previous infections to allow a fast response to future exposures. Vaccines are built to train B and T cells to recognize pathogens without subjecting the body to the other risks of infection.
These studies are part of the international Human Cell Atlas (HCA) consortium. What is a cell atlas, and how can they improve our understanding of the immune system and related diseases?
Dr. Cecilia Domínguez Conde: A cell atlas is a comprehensive map of all the cells that make up a system or organ; for example, both studies looked at the immune system. Both atlases add to the Human Cell Atlas Initiative, which aims to sequence every cell in the human body and create a full reference map with every cell type's position, function, and characteristics, from development to adulthood.
By creating a complete cell atlas, we can identify new cell types that were previously unknown and shed light on what happens when something goes wrong and causes disease. Uncovering more about the cells that underpin disease and how they differ from healthy cells can give insight into treatment and prevention strategies. For example, the findings from our immune cell atlas have a great potential to inform the design of the next generation of vaccination strategies and cell-based therapies.
Regarding the study entitled "Mapping the developing human immune system across organs," how did you conduct your research, and what were your main findings?
Dr. Chenqu Suo: In our research, we created an atlas of the developing human immune system across nine organs by using spatial transcriptomics and single-cell RNA sequencing to map the exact location of specific cells within developing tissues. We found different types of immune cells in the very early stages of organ development compared to those found later on. Some immune cells found in the early stages of the organ suggest that they might help support the developing tissues instead of combating pathogens.
We also identified a new type of B cell, B1, and a distinctive subset of T cells known as unconventional T cells. Both of these new types of cells are found more abundantly in early life compared to adults, suggesting that the immune system at this stage is more geared towards responding quickly to any pathogenic challenge than the adult immune system.
Regarding the study entitled "Cross-tissue immune cell analysis reveals tissue-specific features in humans," how did you conduct your research, and what were your main findings?
Dr. Cecilia Domínguez Conde: Using single-cell transcriptomics, we simultaneously analyzed immune cells across 16 tissues from 12 individual adult organ donors. To perform a systematic annotation of cells across tissues, we built a cross-tissue cell type reference and classifier, called CellTypist, that can now also be used by the broader scientific community. Using a combination of CellTypist and complementary analysis, we have revealed numerous immune cells in the myeloid and lymphoid lineages and their tissue distribution. For example, in the memory T cell compartment, we find various subtypes of effector and resident memory populations and their TCR clonal features.
How do you foresee the findings from these studies influencing and informing the treatment of disease?
Dr. Chenqu Suo: Both immune cell atlases could help pinpoint new therapeutic targets for drug development and cell-based therapies by identifying genes linked to certain diseases, such as certain cancers and autoimmune diseases. An example of this is congenital immunodeficiency, a group of genetic diseases where a child is born without the ability to fight infections. While some genes have been linked to congenital immunodeficiency, the exact mechanisms and cells involved are still unknown. Our cell atlas can help pinpoint which cells are impacted by these genes and at what stage of development they are affected, possibly informing new therapies in the future.
Image Credit: Juan Gaertner/Shutterstock.com
Considering the COVID-19 pandemic and the COVID-19 vaccines, how could an immune cell atlas encompassing different cell types and stages of life help our understanding of vaccine effectiveness?
Dr. Cecilia Domínguez Conde: Vaccinations work by generating immune responses within tissues. Creating an immune cell atlas that maps the entire immune system can uncover a new understanding of how these cells form and interact. This in-depth reference map of the immune system can serve as a framework for understanding the type of memory T or B cells generated by vaccines, allowing researchers to see which cells and genes would be effective targets.
Within the field of Immunology, which currently unanswered questions would you hope to see answered in the near future?
Dr. Chenqu Suo: Our research has characterized new populations of B and T cells, but we need further studies to understand why these are important for the correct development. In addition, we still don't know if these originate from the same progenitors as conventional B and T cells or if they go through an alternative differentiation path. Understanding the origin of these cells will give us more clues as to what environment the immune system needs to grow. This knowledge can develop new ways to culture immune cells in the lab that could be used for therapeutic purposes.
What is next for you and your research?
Dr. Chenqu Suo: I am going back to clinical practice after the Ph.D. and hope to do research alongside my day-to-day clinical work. I wish to utilize the same technologies I've learned in my Ph.D. to study pediatric diseases.
Dr. Cecilia Domínguez Conde: I will be starting my research group at the Genomics Centre of the Human Technopole in Milan to study pediatric immunology and rare diseases affecting immune defense. (https://humantechnopole.it/en/research-groups/dominguez-conde-group/)
Where can readers find more information?
About Dr. Chenqu Suo
Chenqu is a Wellcome Trust-funded clinical Ph.D. student supervised by Dr. Sarah Teichmann. Chenqu completed her medical degree at the University of Cambridge and was a pediatrician in training before joining the lab. Her resear ch interests lie in human immunology, in particular immune system development, in vitro cell engineering, and implications in understanding and managing diseases
ch interests lie in human immunology, in particular immune system development, in vitro cell engineering, and implications in understanding and managing diseases
About Dr. Cecilia Domínguez Conde
Cecilia Domínguez Conde is a Group Leader at the Population & Medical Genomics program of the Genomics Centre at Human Technopole.  After training as a pharmacist at the University of Seville, Cecilia went on to do a Ph.D. in Immunology at the Research Centre for Molecular Medicine (CeMM) in Vienna where her work focused on dissecting the genetic cause of molecularly undiagnosed primary immunodeficiencies using exome sequencing. In 2019 Cecilia joined the Teichmann lab at the Wellcome Sanger Institute where her focus has been to dissect the diversity of human immune cell types across lymphoid and non-lymphoid tissues as part of the Human Cell Atlas initiative. Her research group at HT uses cutting-edge genomic technologies to study developmental immunology.
After training as a pharmacist at the University of Seville, Cecilia went on to do a Ph.D. in Immunology at the Research Centre for Molecular Medicine (CeMM) in Vienna where her work focused on dissecting the genetic cause of molecularly undiagnosed primary immunodeficiencies using exome sequencing. In 2019 Cecilia joined the Teichmann lab at the Wellcome Sanger Institute where her focus has been to dissect the diversity of human immune cell types across lymphoid and non-lymphoid tissues as part of the Human Cell Atlas initiative. Her research group at HT uses cutting-edge genomic technologies to study developmental immunology.