Recent clinical trials investigating the therapeutic potency of sodium-glucose cotransporter 2 inhibitors (SGLT2i) have highlighted that this drug is highly effective in treating patients with cardiovascular and renal diseases. SGLT2 inhibitors are a class of prescription medicines that are FDA-approved for use with diet and exercise to lower blood sugar in adults with type 2 diabetes.
 Study: SGLT2 Inhibitors: The Next Blockbuster Multifaceted Drug? Image Credit: Cagkan Sayin / Shutterstock
Study: SGLT2 Inhibitors: The Next Blockbuster Multifaceted Drug? Image Credit: Cagkan Sayin / Shutterstock
Background
Diabetes is a chronic disease caused by reduced insulin production or impaired insulin activity. Uncontrolled diabetes can increase the risk of cardiovascular and renal abnormalities, with cardiovascular disease being the primary cause of diabetes-related mortality.
Sodium-glucose cotransporter 2 inhibitors (SGLT2i) are anti-diabetic drugs isolated for the first time from an apple tree in 1835. Since then, the drug has been widely used in diabetic research because of its effect on renal glucosuria (excessive loss of glucose through urine) and the ability to inhibit glucose reabsorption.
Role of SGLT2i in diabetes, cardiovascular disease, and renal disease
The US Food and Drug Administration (FDA) mandated the inclusion of cardiovascular outcomes in diabetes clinical trials in 2008.
The earliest clinical trial investing therapeutic effects of SGLT2i (empagliflozin) in type 2 diabetes patients has shown that the drug is highly effective in reducing myocardial infarction, stroke, heart failure-related hospitalization, and cardiovascular death. Since then, numerous studies have demonstrated the effectiveness of SGLT2i in reducing major cardiovascular complications in type 2 diabetes patients.
Clinical trials conducted on heart failure patients without type 2 diabetes have shown that SGLT2i (dapagliflozin) prevents disease progression and reduces disease-related hospitalization and mortality.
Clinical trials conducted in patients with type 2 diabetes and chronic kidney disease have shown that SGLT2i (canagliflozin) effectively prevents end-stage kidney disease development, deterioration of kidney health, and disease-related mortality. In addition, the drug has been found to reduce heart failure-related hospitalization and mortality.
In chronic kidney disease patients with or without diabetes, SGLT2i (dapagliflozin) has been found to reduce disease progression, renal mortality, and cardiovascular mortality.
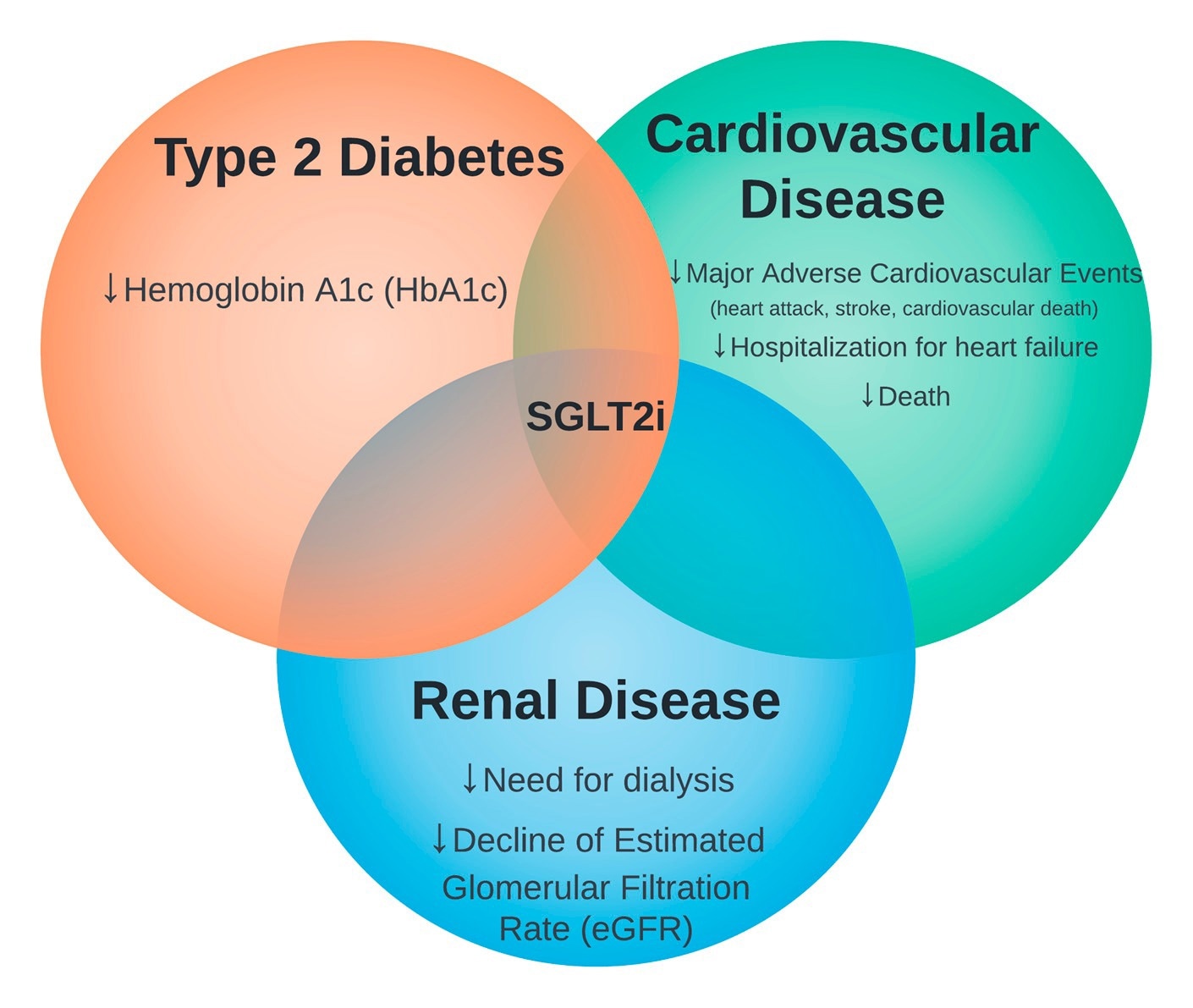
The intersection of sodium-glucose cotransporter 2 inhibitors (SGLT2i) therapy in type 2 diabetes, cardiovascular disease, and renal disease.
Mode of action of SGLT2i
In diabetes, SGLT2i primarily works by inhibiting glucose reabsorption at the renal proximal tubule.
In cardiovascular disease, the drug is believed to work by improving cardiac energetics, reducing blood pressure, preventing inflammation, and diuresis, inhibiting the nervous system, and preventing cardiac remodeling.
In renal disease, it has been hypothesized that the drug reduces intraglomerular pressure by increasing sodium transport through the nephron, leading to adenosine-mediated constriction of afferent glomerular arterioles.
Glucagon-like peptide-1 (GLP-1) receptor agonists are another class of widely used anti-diabetic drugs. The comparison analysis of these two drug classes in diabetic patients indicated that while SGLT2i effectively reduces cardiovascular adversities, renal complications, and heart failure-related hospitalization, GLP-1 receptor agonists are effective for managing cardiovascular events, stroke, and body weight.
Regarding treatment-related adverse effects, SGLT2i can cause genital mycotic infection, and GLP-1 receptor agonists can cause vomiting and gastrointestinal complications.
Application of SGLT2i in diabetes and cardiorenal disease treatment
Therapeutic efficacy and acceptable safety profile make SGLT2i a valuable intervention for managing various health conditions, including diabetes and cardiorenal diseases. However, an estimated glomerular filtration rate under 25 mL/min per 1.73 m2 and type 1 diabetes are the main contradictions of SGLT2i therapy.
In patients with volume depletion, active genital mycotic infections, hypotension, and diabetic ketoacidosis, treatment with SGLT2i should be initiated with proper medical attention.
In type 2 diabetic patients, doses of SGLT2i can be gradually increased after 4 – 12 weeks to achieve optimal blood glucose management. If the patients are on insulin or sulfonylurea and have HbA1c under 7.5%, about a 10 to 20% dose reduction in insulin and a 50% dose reduction in sulfonylurea can be considered.
SGLT2i is now considered first-line therapy in heart failure. In hospitalized patients with heart failure, immediate initiation of SGLT2i therapy can be effective in preventing future complications. Such therapy can be initiated at any left ventricular ejection fraction.
In patients with type 2 diabetes and chronic kidney disease, metformin and SGLT2i are considered first-line therapy. Even if the patients have well-controlled blood glucose levels, SGLT2i therapy is still recommended to prevent the progression of cardiorenal diseases.