Obesity has become a pandemic health condition worldwide because of its ever-increasing prevalence. An individual with a body mass index (BMI) equal to or over 30 kg/m2 is considered obese. It is now well-established that obesity significantly increases the risk of cardiovascular diseases, metabolic diseases, and certain cancers.
Infants born to obese mothers also risk developing cardiovascular and metabolic diseases. Gestational diabetes (diabetes developed during pregnancy) is another potential risk factor for the onset of cardiometabolic diseases in infants.
In the current study, scientists have determined the epigenetic landscape of infants born to obese mothers with or without gestational diabetes to understand how these risk factors may influence the health of infants during their first months of life.
Study design
Whole blood samples isolated from 26 infants born to mothers with obesity and gestational diabetes were subjected to genome-wide analysis to identify DNA methylation changes associated with adverse health outcomes.
Longitudinal assessments of the epigenetic landscapes were conducted during the first year of life, specifically at 0, 6, and 12 months. More than 770,000 CpG sites were profiled using Illumina Infinium Methylation EPIC BeadChip arrays.
DNA methylation landscape
The profiling of differentially methylated CpG sites revealed that about 54% of the sites are hypomethylated, and 46% were hypermethylated during the first 6 months of life. However, the complete disappearance of this balance was observed from 6 months to 12 months, with 83% of the sites being hypomethylated and only 17% being hypermethylated.
DNA methylation changes occurred during the first 6 months of life had the most significant impact on epigenetic remodeling. A significant proportion of these changes continued to change from 6 months to 12 months. In both periods of life, these common changes completely preserved their direction of change, indicating that these changes are vital for the optimal development of infants after birth.
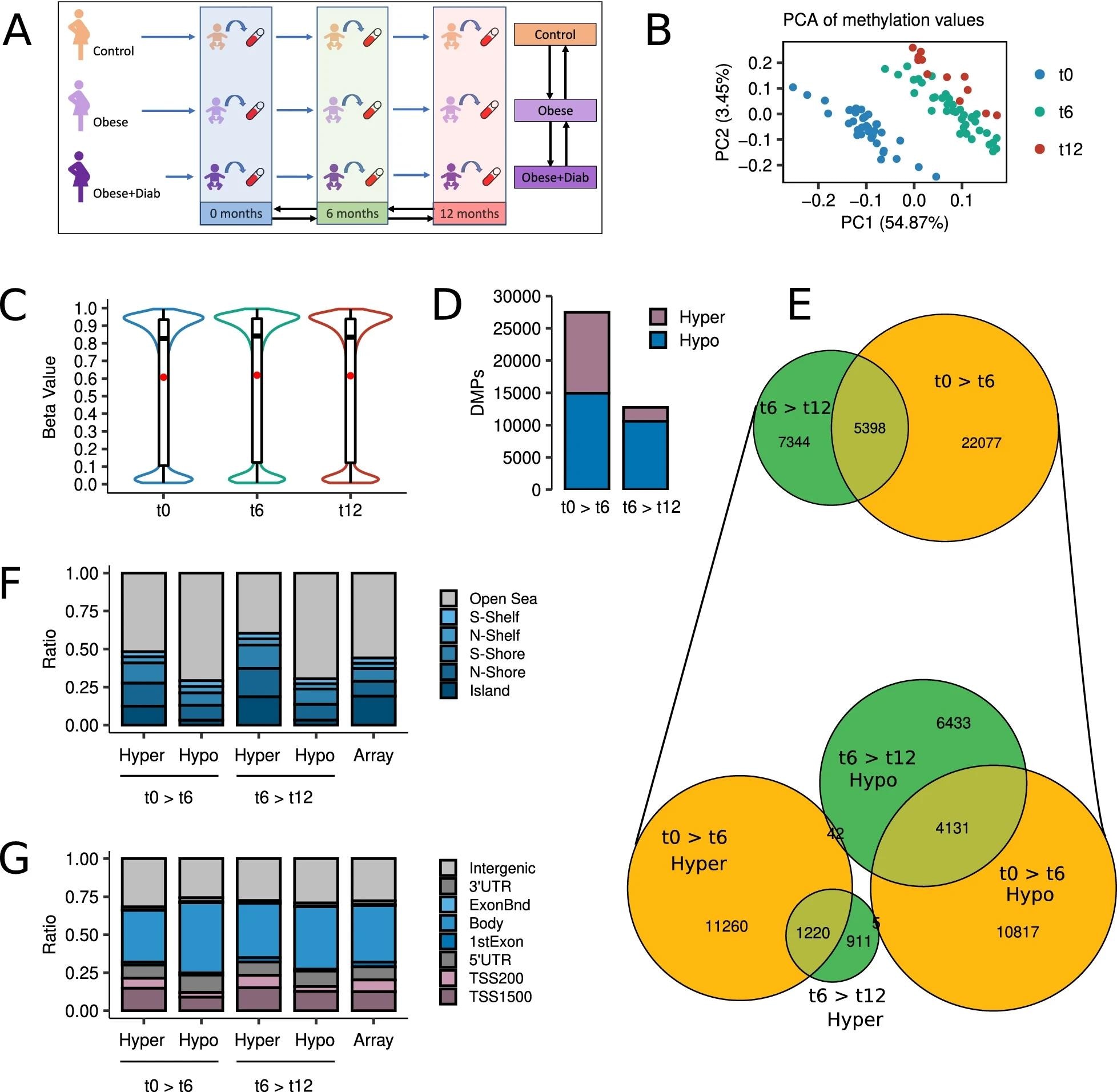 Development is the main origin of methylation changes during the first year of life. a Schematic of the study design. b Scatter plot showing the PCA of the subjects according to their methylation values at the top 100,000 most variable CpG sites. c Violin plots describing the distribution of methylation values at the 772,088 CpGs analyzed by time point. d Barplots depicting the number of hyper- and hypomethylated DMPs (FDR < 0.05) found in 0 > 6 and 6 > 12 longitudinal comparisons. e At the top, a Venn diagram showing the intersections between 0 > 6 and 6 > 12 DMPs. At the bottom, a Venn diagram describing the intersection between hyper- and hypomethylated DMPs at 0 > 6 and 6 > 12 comparisons. f Barplots showing the relative distribution of hyper- and hypomethylated DMPs at 0 > 6 and 6 > 12 comparisons according to their CpG island location status. g Barplots showing the relative distribution of hyper- and hypomethylated DMPs at 0 > 6 and 6 > 12 comparisons according to their gene location status. The rightmost bars represent the background distribution considering all 772,088 probes analyzed.
Development is the main origin of methylation changes during the first year of life. a Schematic of the study design. b Scatter plot showing the PCA of the subjects according to their methylation values at the top 100,000 most variable CpG sites. c Violin plots describing the distribution of methylation values at the 772,088 CpGs analyzed by time point. d Barplots depicting the number of hyper- and hypomethylated DMPs (FDR < 0.05) found in 0 > 6 and 6 > 12 longitudinal comparisons. e At the top, a Venn diagram showing the intersections between 0 > 6 and 6 > 12 DMPs. At the bottom, a Venn diagram describing the intersection between hyper- and hypomethylated DMPs at 0 > 6 and 6 > 12 comparisons. f Barplots showing the relative distribution of hyper- and hypomethylated DMPs at 0 > 6 and 6 > 12 comparisons according to their CpG island location status. g Barplots showing the relative distribution of hyper- and hypomethylated DMPs at 0 > 6 and 6 > 12 comparisons according to their gene location status. The rightmost bars represent the background distribution considering all 772,088 probes analyzed.
Functional dynamics of DNA methylation landscape
The functional analysis of differentially methylated sites identified 6 distinct clusters with varied numbers and sizes of annotated genes.
The hypermethylated sites from cluster 2 were found to be associated with various developmental processes. In contrast, hypomethylated sites from clusters 1 and 5 were found to be associated with immune system activation and maturation pathways.
Impact of maternal obesity and gestational diabetes
To understand the impact of maternal conditions on an infant’s epigenetic changes post-birth, a group of infants born to obese mothers, obese and gestationally diabetic mothers, or healthy mothers was selected to analyze differentially methylated sites.
The findings revealed significant enrichments in shared differentially methylated sites across all the comparison groups. The comparison groups also observed a significant overlap in differentially methylated genomic regions. By separating hypo- and hyper-differentially methylated regions, it was observed that gestational diabetes also induces DNA methylation changes that are not related to obesity.
The cross-sectional analysis of DNA methylation changes revealed that maternal obesity-related hypermethylated regions are concentrated at genes involved in fatty acid transport and vitamin and steroid metabolism (developmental processes). In contrast, hypomethylated regions are concentrated in genes involved in mitochondrial bioenergetics.
Overall, these observations indicate that maternal obesity during pregnancy can alter the development of infants after birth. Considering both risk factors (obesity and diabetes), a strong association was observed between the development-related methylation changes and maternal obesity with gestational diabetes.
Study significance
The study describes DNA methylation landscapes in infants born to obese mothers with or without gestational diabetes. In addition, the study identifies specific DNA methylation biomarkers maintained during the first year of life. These biomarkers at both single CpG positions and genomic regions could potentially differentiate the infants born to mothers with obesity and gestational diabetes from those born to healthy mothers.