To date, no effective therapy is available for patients with thyroid carcinoma (TC). In most cases, TC metastases fail to express the sodium/iodine symporter (NIS) and do not respond to radioactive iodine, an efficient non-invasive treatment for TC. Hence, there is a need to develop experimental animal models to elucidate the underlying mechanism of tumorigenesis and metastasis formation, particularly to formulate therapies for patients resistant to radioiodine therapy.
 Study: Recapitulating thyroid cancer histotypes through engineering embryonic stem cells. Image Credit: Meletios Verras / Shutterstock
Study: Recapitulating thyroid cancer histotypes through engineering embryonic stem cells. Image Credit: Meletios Verras / Shutterstock
Background
Typically, follicular cell-derived TCs are developed from impaired endodermal layer-derived follicular epithelial cells, consisting of histologically differentiated and undifferentiated subtypes. The differentiated TCs include common papillary (PTC) and follicular (FTC) TCs. In most cases, differentiated TCs are indolent tumors with favorable outcomes. However, in some cases differentiated TCs showed high relapse risk and eventually led to death.
Anaplastic TC (ATC) manifests aggressive behavior by invading adjacent tissues and metastasizing distant organs. This type of TC represents around 2% of all TC cases. ATC is either generated from the follicular thyroid epithelium gland by removing biological features linked to iodine uptake or via the de-differentiation process of a pre-existing differentiated TC containing multiple mutations.
Although several studies have uncovered multiple important aspects of TCs, the cell subpopulation in the lineage hierarchy associated with the cell of origin for various TC histotypes, post accumulation of somatic mutations, is poorly understood. Scientists have developed several carcinogenesis models based on different follicular histotypes of cell-derived TCs possessing specific behavior. These models were used to better understand the cell of origin. The major shortcomings of these models are that they fail to determine the phenotypic and genetic intertumoral heterogeneity of follicular cell-derived TCs.
In mammals, tissue repair and homeostasis are associated with tissue-specific stem cells that can self-restore and differentiate. Studies on the hierarchical organization of adult tissue have played an important role in decelerating the process of aging, recovery from injury, and preventing cells from accumulating damage that can ultimately cause cancer.
A model based on progenitor cells introduced the concept of tumorigenesis. This model assumed stem cells to be the source of tumor formation based on their longevity and self-restoration capacity, which is essential for accumulating mutations. To better understand the multiple histopathology and behavior, it is vital to develop a genetic mutation model comprising multiple mutations within the same “target cell.”
Even though researchers have successfully determined a subpopulation of cancer stem cells (CSCs)/progenitor cells associated with TC initiation, their alteration phase in the hierarchy lineage is unclear.
About the Study
The oncogenic events that take place in tissue-resident stem cells/progenitor cells during TC transformation were effectively reproduced in this study. Furthermore, the genetic mutations of the tissue-resident progenitor cells were linked to the evolutionary process of thyroid tumorigenesis. Therefore, the current research elevated the understanding related to TC incidence rate based on the presence of stem/progenitor cells in the thyroid gland.
Different TC histotypes were recapitulated based on stimulation of specific genomic changes introduced by CRISPR-Cas9 in hESC-derived thyroid progenitor cells (TPCs) at day 22. CRISPR/Cas9 technology has been previously used to introduce oncogenic driver mutations in breast, colon, or pancreas organoids and induce tumor transformation.
The common genetic and epigenetic changes linked to TC have been discovered previously. These studies identified two main signaling pathways, namely, the MAPK and PI3K/AKT/mTOR signaling pathways, related to TC, which induce uncontrolled cell division, longevity, and cell migration.
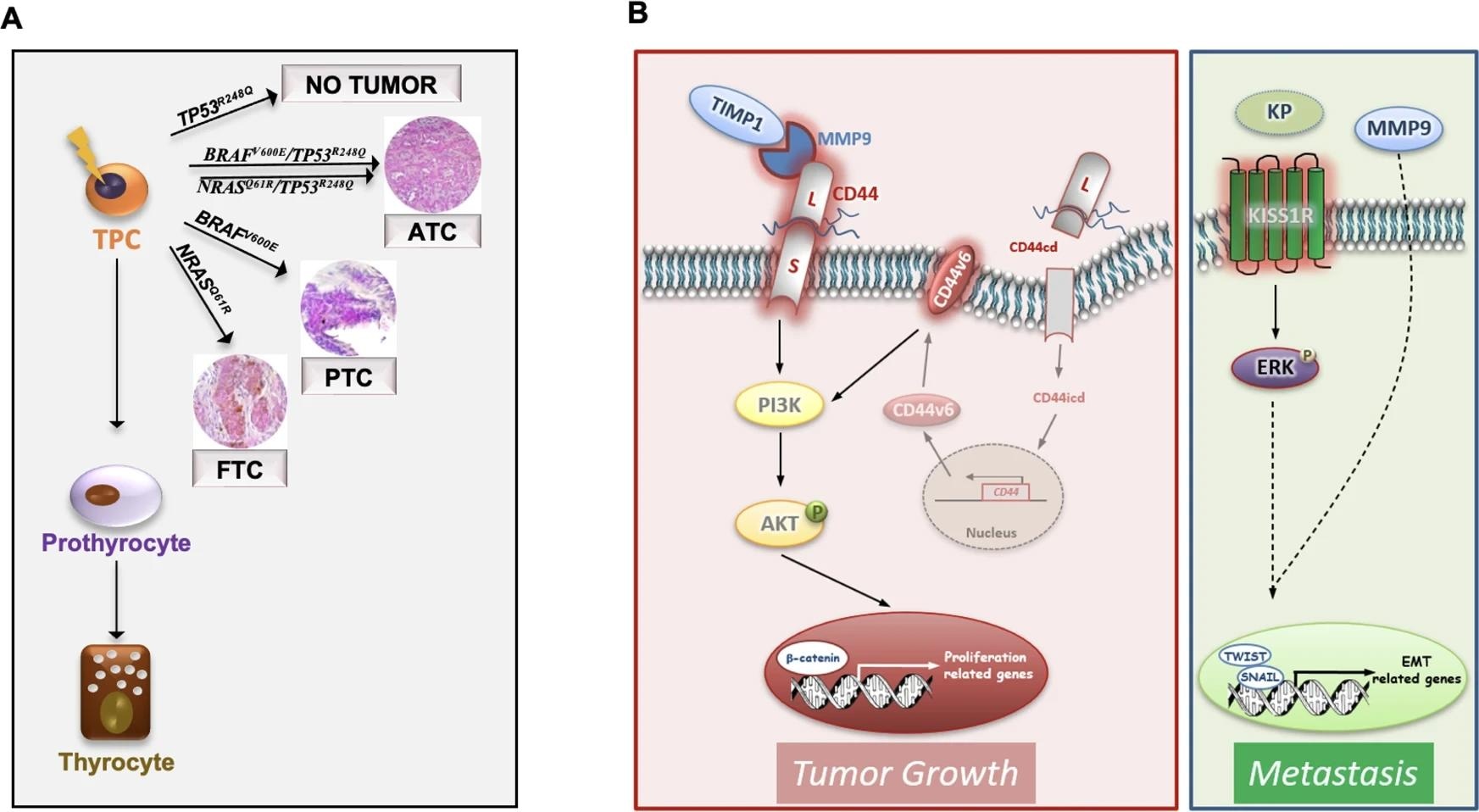 A Schematic model illustrating that the most common TC genetic alterations (BRAFV600E, NRASQ61R, BRAFV600E/TP53R248Q and NRASQ61R/TP53R248Q) in thyroid progenitor cell (TPC) recapitulate the different TC histotypes (FTC, PTC and ATC). Of note TP53R248Q alone is not required for TC initiation. B Model of ternary complex (TIMP1/MMP9/CD44) and KISS1R driven pathways in engineered D22 TPCs. TIMP1 and pro-MMP9 complex formation activates MMP9 and consequently leads to the cleavage of CD44. The CD44 intracytoplasmic domain (CD44icd) translocates into the nucleus where it induces CD44v6 transcription. CD44v6 promotes TPC proliferation through PI3K/AKT pathway. The binding of kisspeptins (KP) to KISS1R activates ERK and cooperates with MMP9 to promote the transcription of EMT-related genes, including TWIST and SNAIL, driving metastatic engraftment.
A Schematic model illustrating that the most common TC genetic alterations (BRAFV600E, NRASQ61R, BRAFV600E/TP53R248Q and NRASQ61R/TP53R248Q) in thyroid progenitor cell (TPC) recapitulate the different TC histotypes (FTC, PTC and ATC). Of note TP53R248Q alone is not required for TC initiation. B Model of ternary complex (TIMP1/MMP9/CD44) and KISS1R driven pathways in engineered D22 TPCs. TIMP1 and pro-MMP9 complex formation activates MMP9 and consequently leads to the cleavage of CD44. The CD44 intracytoplasmic domain (CD44icd) translocates into the nucleus where it induces CD44v6 transcription. CD44v6 promotes TPC proliferation through PI3K/AKT pathway. The binding of kisspeptins (KP) to KISS1R activates ERK and cooperates with MMP9 to promote the transcription of EMT-related genes, including TWIST and SNAIL, driving metastatic engraftment.
Earlier studies revealed that BRAF is mutated in ~40% of PTCs and ~35% of FTCs, and ~55% of ATCs. The current study found BRAFV600E or NRASQ61R recapitulates PTC and FTC histotypes, respectively, at morphological, transcriptomics, and phonotypic levels.
Consistent with previous studies, the current study revealed that TP53 mutation alone could not drive TC tumorigenesis. However, in association with BRAFV600E or NRASQ61R, it can produce undifferentiated TCs enriched with metastatic-associated gene signatures that manifest the aggressive behavior of the cell in ATC.
Transcriptomic analyses of metastatic TC lesions of human patients along with xenografts from TPCs containing BRAFV600E or NRASQ61R in combination with TP53R248Q mutations indicated the emergence of the TIMP1/MMP9/CD44 complex. This complex plays an important role in TC promotion. It was also observed that activation of KISS1/KISS1R signaling is essential for metastatic growth. Notably, poor TC prognosis was linked to a high level of KISS1/KISS1R expression. This could be a promising marker to analyze the therapeutic efficacy in advanced TC patients.
Conclusions
KISS1R and TIMP1 targets could be considered in the future as adjuvant therapy to sensitize ATCs. Future studies can utilize the newly developed experimental model, based on the genetically engineered hESC-derived cells, to investigate genes and pathways that promote therapy resistance. In addition, this model could be used to study the tumorigenesis mechanisms in different organs.