Sponsored Content by Cerba ResearchReviewed by Louis CastelMar 16 2023
NewsMedical speaks to Cerba Research on how to achieve immune cell checkpoint profiling of solid tumors using multiplex immunofluorescence.
Could you please provide our readers with a brief introduction to immune cell checkpoint profiling?
Immune checkpoint proteins as regulators are crucial for managing self-tolerance and enabling cancer cells to bypass immune destruction.
Checkpoint inhibitor (CKI) blockade therapies can help restore antitumoral immunity. Combination blockade has demonstrated the potential of more significant tumor growth inhibition than monotherapies in preclinical studies.
Multiplex immunofluorescence offers a technical advantage by enabling the detection of co-expression and spatial organization of multiple targets within a preserved tissue architecture on a single slide. Cerba Research has developed the HISTOPROFILE®-CKI multiplex immunohistochemistry panel to offer personalized immune cell checkpoint profiling.
What is Multiplex Design and Validation?
Sequential multiplex protocol with Opal® (Akoya Biosciences) fluorophores was carried out on the BOND RX (Leica) slide stainer. The multiplex panel was then tested on healthy human tonsils and prostate, liver, lung, and skin tumors. Whole slide multispectral images were then acquired with the VECTRA ® PolarisTM (Akoya Biosciences) slide scanner for validation requirements.
What analysis methods are applied during a typical study to achieve validation?
The images we acquire are usually analyzed with INFORM® (Akoya Biosciences) or HALO® (Indica Labs) Highplex module. Once we have validated a base panel comprised of CD3, CD8, programmed cell death 1, programmed death-ligand 1, and DAPI (human tonsil, scale bar = 100 µm), the position of interest (Px) in the multiplex is compared to the reference (simplex IHC) protocol (P1). Then, the percentage of the positive area was compared between the two protocols to validate the panel. To assess concordance, HALO is used, allowing for validation of the complete multiplex panel.
How do you determine the reproducibility of the results?
Over three days, three different operators experimented using the Histoprofile®-CKI multiplex panel. This approach visually demonstrated equivalent staining for all targets. Each target was then analyzed on three whole scans using HALO. The results revealed equivalent staining profiles for each target.
How do you establish the validity of the results?
The Histoprofile®-CKI multiplex panel with IDO was tested for robustness and each representative target – CD3, CD8, PD-1, PD-L1, IDO, and DAPI were detected in lung adenocarcinoma, hepatocellular carcinoma, prostate adenocarcinoma, and melanoma. This helped establish the validity of the results.
Can you describe how the approach serves to modify the immune checkpoint target?
The targets TIGIT, LAG-3, and CD47 can be readily applied to the base panels CD3, CD8, PD-1, PD-L1, and DAPI, which demonstrates the flexibility of Histalim’s Histoprofile®-CKI multiplex panel.
The flexible nature of the method facilitates interchangeability of the immune checkpoint targets, which translates as the ability to modify the application across multiple tissue types.
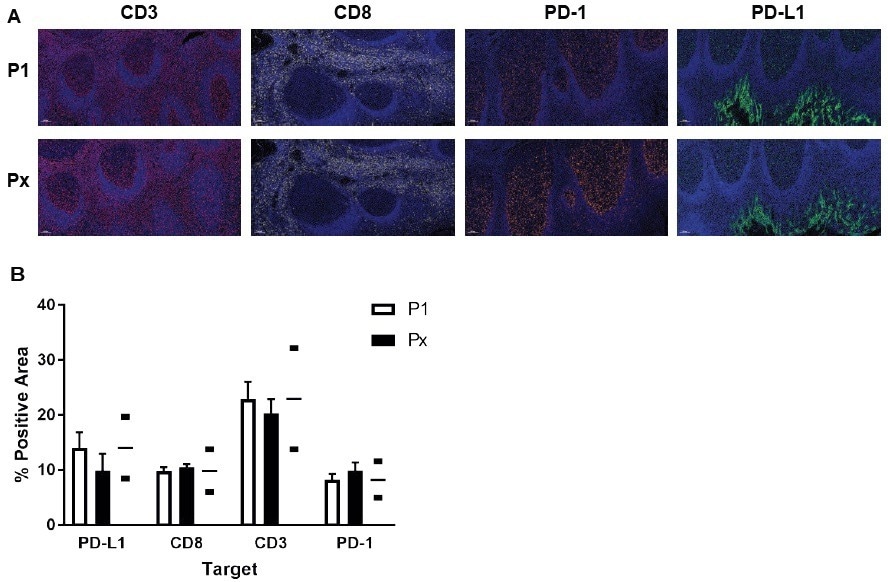
Base HISTOPROFILE®-CKI Panel : A) We have validated a base panel, comprised of CD3 (red), CD8 (yellow), programmed cell death 1 (PD-1, orange), programmed death-ligand 1 (PD-L1, green), and DAPI (blue) (human tonsil, scale bar = 100 μm). For each marker, the position of interest (Px) in the multiplex was compared to the reference (simplex IHC) protocol (P1). B) The % of positive area was compared between the two protocols to validate the panel. Image Credit: Cerba Research
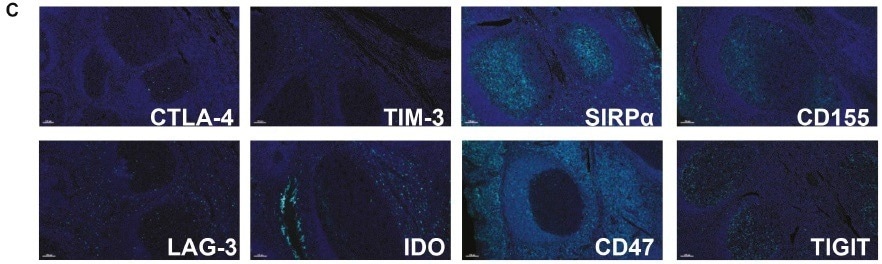
Image Credit: Cerba Research
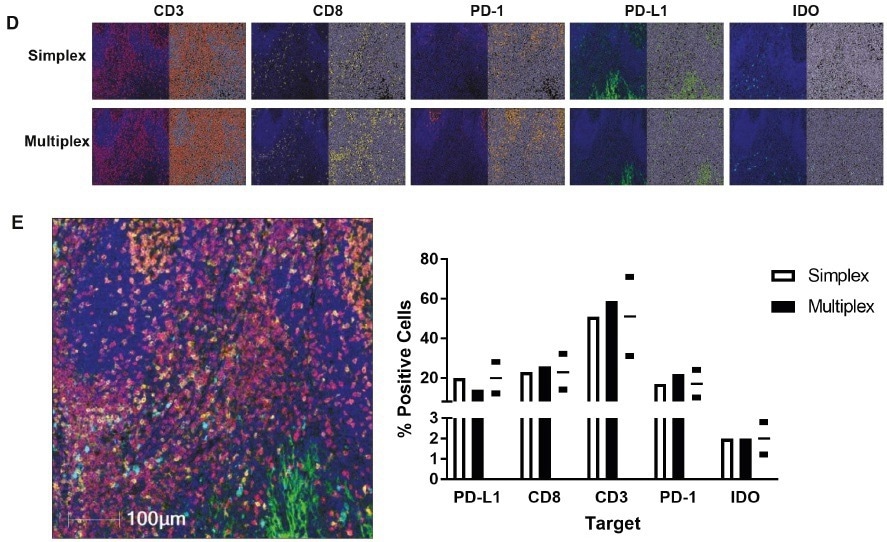
Image Credit: Cerba Research
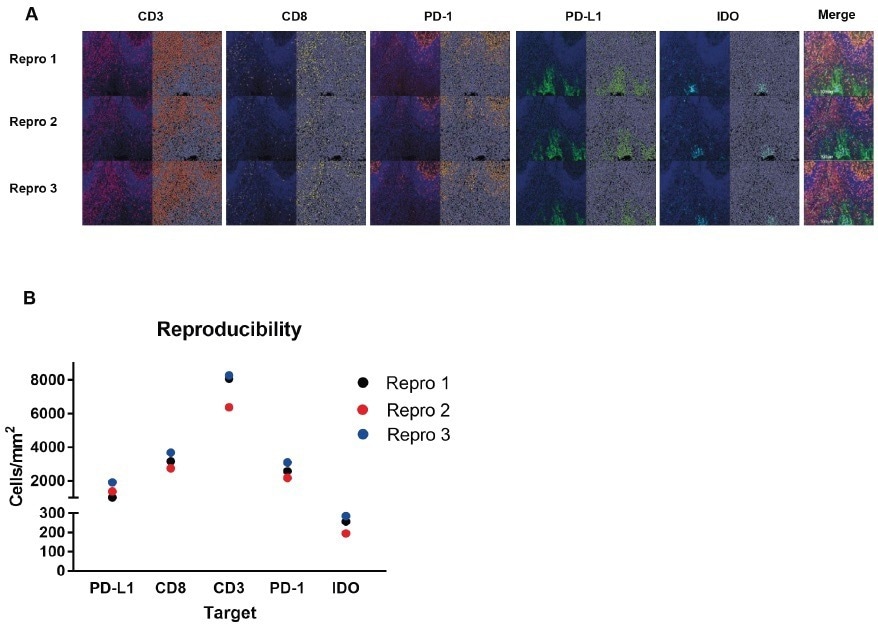
Image Credit: Cerba Research
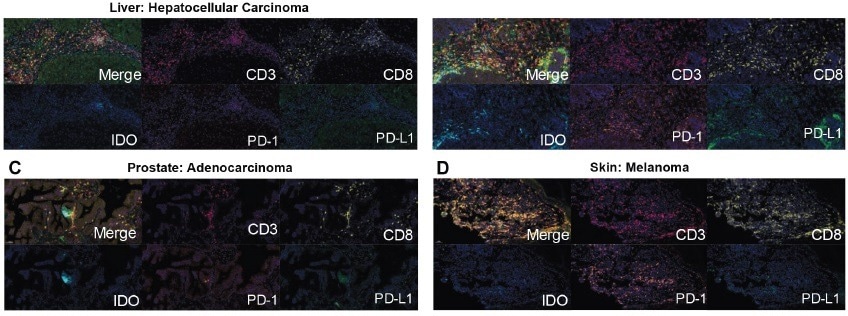
Image Credit: Cerba Research
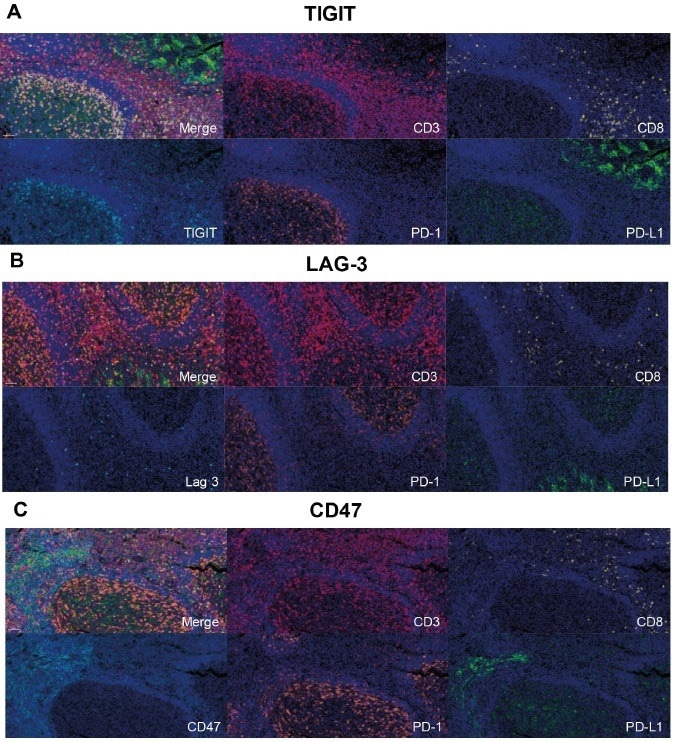
Image Credit: Cerba Researc
About Cerba Research
For over 35 years, Cerba Research has been setting the industry standard for exemplary clinical trial conduct. Today, across five continents, with a focus on precision medicine, we are changing the paradigm of the central lab’s role in complex clinical research.
From protocol inception through development and to market, our passionate experts deliver the highest quality specialized and personalized laboratory and diagnostic solutions. Partner with us for the most efficient strategy to actualize your biotech and pharmaceutical products sooner and improve the lives of patients worldwide.