Microbial infections are caused by harmful bacteria, fungi, or viruses and affect millions worldwide. Increased antimicrobial resistance (AMR) has been observed globally, threatening the healthcare system. Among AMR, antifungal resistance has been chiefly found among immunocompromised patients.
 Study: Identification and Elimination of Antifungal Tolerance in Candida auris. Image Credit: Kateryna Kon Shutterstock
Study: Identification and Elimination of Antifungal Tolerance in Candida auris. Image Credit: Kateryna Kon Shutterstock
Background
Fungi thrive at warmer temperatures, and climatic changes have aided their growth. As a result, a continual increase in the global rates of fungal infections has been predicted. Scientists are particularly concerned about antifungal resistance because of the limited number of classes of antifungal drugs. Surprisingly, no new class of antifungal drugs has reached the market for over a decade.
One of the most common fungal infections is caused by Candida yeast species. Clinicians have shown particular concern about the fungal infection caused by C. auris because of its resistance to antifungal drugs. This fungal pathogen is found in more than 47 countries worldwide and has a mortality rate of up to 45% among patients with bloodstream infections.
C. auris has shown multidrug resistance. When a particular fungus is resistant to more than one in three or more antimicrobials, it is known as multidrug-resistant. Notably, in some cases, C. auris has also shown pan-drug resistance, where it was found to be non-susceptible to drugs belonging to all antimicrobial classes.
It is important to note the difference between antifungal tolerance and antifungal resistance. The antifungal resistance results from heritable genetic changes, which allows the fungal cell to grow above the minimum inhibitory concentration (MIC) in a concentration-dependent manner. However, antifungal tolerance results from phenotypic heterogeneity, a reversible phenomenon. Tolerant cells slowly grow above MIC.
It is essential to understand the mechanisms associated with tolerance in Candida species. Previous studies have indicated that multiple factors, such as aneuploidy, are associated with tolerance in Candida species. Although Hsp90-facilitated azole tolerance has been found in C. auris, it is unclear whether this fungal species is tolerant to non-azole classes of antifungal drugs.
Currently, no clinical assays are available to identify antifungal tolerance, which could be highly beneficial in determining the effectiveness of mono- and combination-antifungal therapies.
About the Study
This study used broth microdilution and disk diffusion assays, along with diskImageR, to evaluate the fungal tolerance to different classes of antifungal drugs. In addition, analyses were conducted to test whether the fungal tolerance could be removed via adjuvant antifungal therapy.
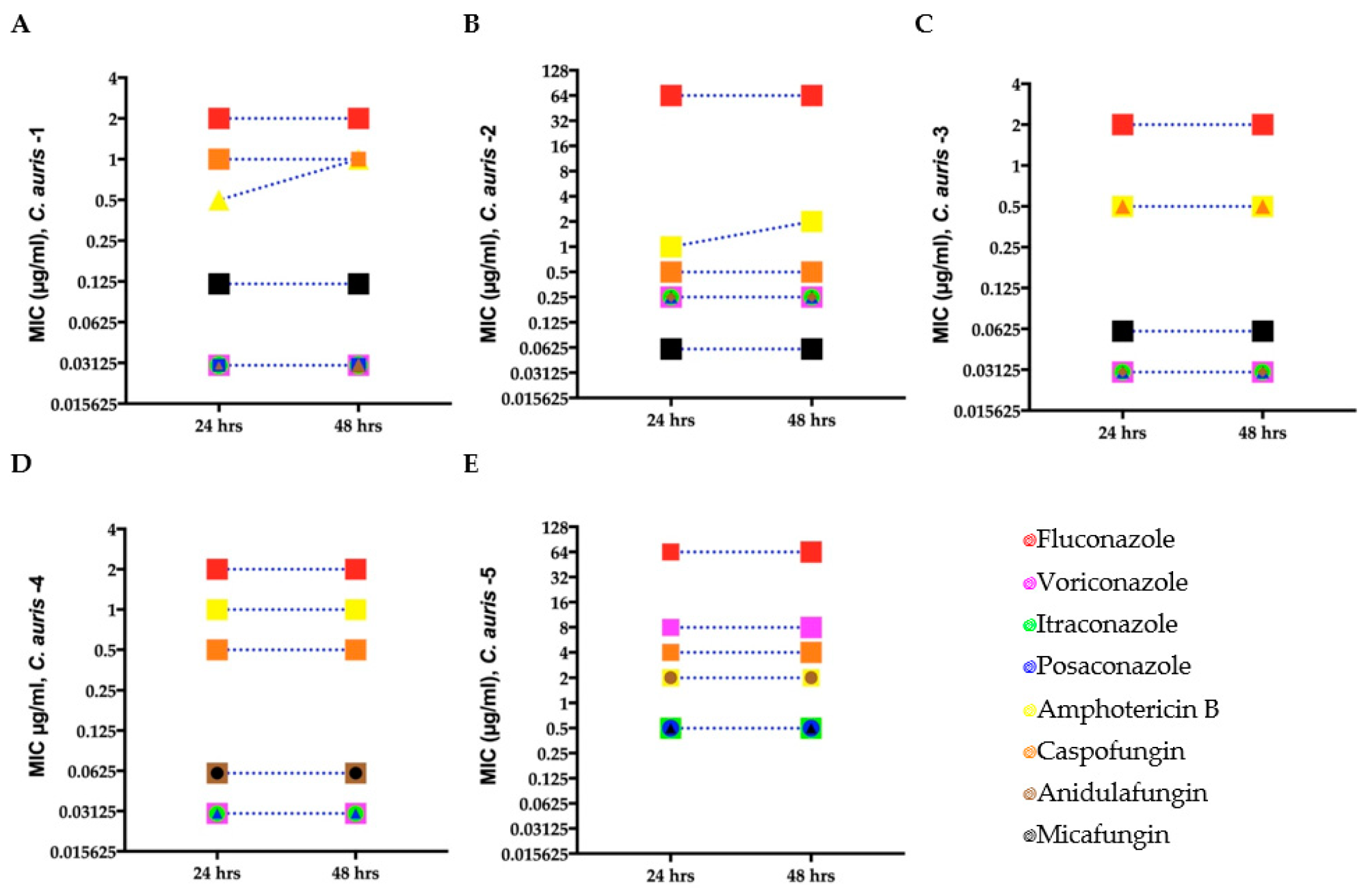 Minimum inhibitory concentration (MIC) for clinical C. auris isolates (A–E) growing in antifungal microwell plates to determine susceptibility/resistance to antifungal drugs. Mean MICs of five clinical C. auris isolates measured after 24 and 48 h for four fungistatic drugs (fluconazole, itraconazole, posaconazole, and voriconazole) and two fungicidal drugs (amphotericin B and caspofungin). Different symbols denote C. auris isolates with the same MIC.
Minimum inhibitory concentration (MIC) for clinical C. auris isolates (A–E) growing in antifungal microwell plates to determine susceptibility/resistance to antifungal drugs. Mean MICs of five clinical C. auris isolates measured after 24 and 48 h for four fungistatic drugs (fluconazole, itraconazole, posaconazole, and voriconazole) and two fungicidal drugs (amphotericin B and caspofungin). Different symbols denote C. auris isolates with the same MIC.
MIC for each C. auris isolate was determined using broth microdilution assays. Antifungal tolerance of C. auris was analyzed against different classes of antifungal drugs, such as fluconazole, amphotericin B, itraconazole, anidulafungin, voriconazole micafungin, caspofungin, and posaconazole.
Images of each disk diffusion plate were obtained after 24 hours and 48 hours at maximum resolution. Fungal tolerance was quantified using supra-MIC growth from microbroth dilution assays and the fraction of growth (FoG) in the zone of inhibition (ZOI) from disk diffusion assays.
The authors also analyzed the effectiveness of combination therapy using an antifungal agent and adjuvant (chloroquine) against C. auris, C. parapsilosis, and Issatchenkia orientalis.
Study Findings
A limited number of clinical C. auris isolates were tolerant to fungistatic drugs, such as fluconazole, voriconazole, itraconazole, and posaconazole, and fungicidal drugs, e.g., amphotericin B and caspofungin. Azole tolerance was also found in C. parapsilosis; however, I. orientalis remained resistant to fluconazole.
Tolerance was detected after 24 hours and 48 hours by FoG and was documented using diskImageR and ImageJ. This finding indicates that a specific subpopulation of C. auris is non-susceptible to different antifungal agents.
Importantly, this tolerance quantification method could be used by clinical diagnostic laboratories along with the standard testing for antifungal susceptibility/resistance to formulate better antifungal strategies.
The tolerant fungal cells that grew inside ZOI upon subculture were significantly less than the parental population, indicating the presence of phenotypic heterogeneity instead of genetic variation.
In the future, the underlying mechanism associated with C. auris tolerance must be studied. Notably, the authors observed reduced or eliminated tolerance in some C. auris isolates by combining antifungal drugs (azole, polyene, and echinocandin) with the antimalarial drug (chloroquine).
Conclusions
C. auris was tolerant to several antifungal drugs, such as caspofungin, fluconazole, posaconazole, voriconazole, itraconazole, and amphotericin B. Fungal tolerance is a reversible phenomenon, which could be detected at 24 hours and 48 hours.
For the first time, this study has demonstrated that combining antifungal drugs with adjuvant chloroquine can eliminate tolerance and resistance in C. auris. However, the findings must be validated using in vitro and in vivo experiments.