The traditional Chinese medicine qiliqiangxin reduces hospitalization for heart failure and cardiovascular death in patients with heart failure and a reduced ejection fraction (HFrEF), according to late breaking research presented in a Hot Line session today at ESC Congress 2023.
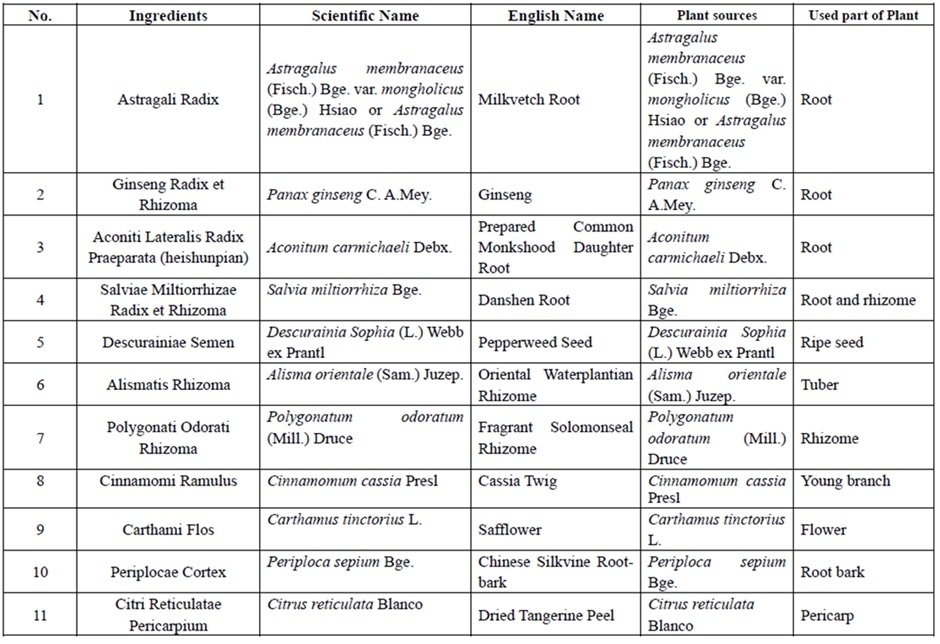
Qiliqiangxin is a traditional Chinese medicine extract obtained from 11 types of herbs (Table 1). In a pilot study, qiliqiangxin reduced N-terminal pro-B-type natriuretic peptide (NT-proBNP) levels and improved heart failure symptoms in patients with HFrEF when added to established heart failure treatment. Preclinical studies have also indicated that qiliqiangxin has beneficial effects on attenuating myocardial fibrosis and cardiac remodeling.
The QUEST trial evaluated the clinical efficacy and safety of qiliqiangxin on major heart failure outcomes in HFrEF patients. The trial was conducted at 133 hospitals in mainland China and Hong Kong SAR of China.
The trial enrolled adult HFrEF patients with a left ventricular ejection fraction of 40% or below and NT-proBNP of 450 pg/ml or higher who had been on a stable standardized baseline treatment regimen for at least two weeks prior to enrolment. Patients were randomized in a 1:1 fashion to receive qiliqiangxin (four capsules, three times daily) or placebo on top of standard medications for chronic heart failure. The primary endpoint was a composite of rehospitalization for worsening heart failure or cardiovascular death.
A total of 3,110 patients were included in the analysis, with 1,555 randomized to qiliqiangxin and 1,555 randomized to placebo. The average age was 62 years and 72.1% were men. At baseline, the mean left ventricular ejection fraction was 32%, and the median NT-proBNP was 1730.80 pg/ml.
During a median follow-up of 18.3 months, the primary endpoint occurred in 389 patients (25.02%) in the qiliqiangxin group and in 467 patients (30.03%) in the placebo group (hazard ratio [HR], 0.78; 95% confidence interval [CI], 0.68 to 0.90; p<0.001). This effect was related to both lower risks of rehospitalization for worsening heart failure (HR, 0.76; 95% CI, 0.64 to 0.90; p=0.002) and cardiovascular death (HR, 0.83; 95% CI, 0.68 to 0.996; p=0.045) in the qiliqiangxin group. The effect of qiliqiangxin on the primary outcome was generally consistent across prespecified subgroups including in the subgroups defined according to age and NT-proBNP level, and in patients with or without angiotensin receptor/neprilysin inhibitors (ARNIs).
In terms of secondary endpoints, the decrease in serum NT-proBNP between baseline and three-month follow-up was greater in the qiliqiangxin group (-444.00 [interquartile range -1401.00 to 85.00]) than in the placebo group (-363.00 [interquartile range -1280.00 to 183.00]) (p=0.047), which was consist with the previous pilot study.
Analysis of safety endpoints demonstrated no significant difference in all-cause mortality, which occurred in 221 patients (14.21%) in the qiliqiangxin group and 262 patients (16.85%) in the placebo group (HR, 0.84; 95% CI, 0.70 to 1.01; p=0.058). Qiliqiangxin capsules were well-tolerated, with no major differences between the two groups in adverse events including gastrointestinal symptoms, worsening renal function and increased liver enzymes.
To our knowledge, this was the first randomized, double-blind controlled trial of a traditional Chinese medicine for the treatment of chronic heart failure. Our findings demonstrate meaningful clinical benefit with qiliqiangxin in patients with HFrEF, which support the use of qiliqiangxin as an adjunct therapy for treating heart failure."
Xinli Li, Principal Investigator Professor, First Affiliated Hospital of Nanjing Medical University, Nanjing, China
Table 1: Ingredients of qiliqiangxin