Cell Signaling Technology (CST), a life science discovery technology company and leading provider of antibodies, kits, and services, today announced the launch of its SignalStar Multiplex IHC technology, a revolutionary new tool for spatial biology research using mid-plex, high-throughput immunohistochemistry (IHC) assays.
The SignalStar technology labels up to eight targets in formalin-fixed, paraffin-embedded (FFPE) tissues simultaneously with flexible, highly validated antibody panels ready to interrogate cellular presence, location, function, and biomarker coexpression patterns. The SignalStar Multiplex IHC assay uses a proprietary technology to amplify the antibody signals, allowing for the detection of targets with low expression levels for up to eight proteins in a single tissue.
These insights are essential for understanding how cells organize and interact to influence the tissue microenvironment (TME) and drive disease progression and therapeutic response.
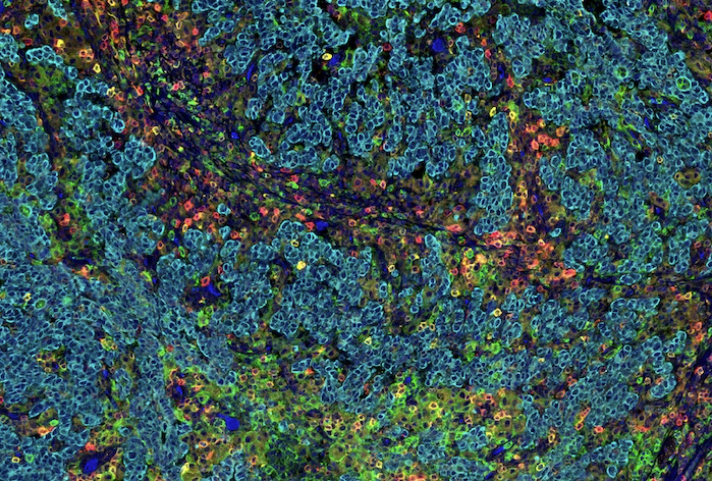
A SignalStar Multiplex IHC assay analysis of human gastric adenocarcinoma. Staining was performed on the BOND RX Fully Automated Research Stainer by Leica Biosystems
“With the SignalStar technology, reliable, reproducible spatial biology data can be generated in just two days,” said Sarah Klein, Associate Director of Multiplex Assay Development at CST. “Additionally, as research changes and evolves or new targets are identified, the SignalStar antibodies and fluorophores are interchangeable, allowing researchers to switch out targets and quickly redesign panels without the need for additional panel or protocol optimization. This unparalleled flexibility delivers results up to 70% faster than some other mIHC methods.”
Researchers can take advantage of the online SignalStar Multiplex IHC Panel Builder to easily design experiments. The panel builder enables the selection of targets from a menu of rigorously validated antibodies that work right out of the box, significantly reducing panel and protocol optimization time. On average, panel optimization time for 8-plex assays is reduced from six months to zero days, while validated manual and automated protocols can reduce protocol time from seven days to two days. Ready-to-go, customized kits built on the SignalStar Multiplex IHC Panel Builder are shipped within two business days.
“Every SignalStar antibody is rigorously validated to work in any combination in SignalStar assays, and just like all CST antibodies, are guaranteed to perform as expected,” said Katie Crosby, Sr. Director, Antibody Applications & Validation at CST. “Researchers can confidently analyze limited, precious FFPE tissue samples knowing that they’re maximizing data acquisition from each sample and getting reliable, reproducible results, the first time and every time.”
Utilizing the SignalStar Multiplex IHC technology every antibody is validated against the chromogenic gold standard and is consistently reproducible across replicates. Protocols demonstrate reproducibility across different antibody combinations, experiments, and imaging instruments. Stable fluorescent signals with distinct emission peaks make SignalStar Multiplex IHC technology panels compatible with most fluorescent imaging instrumentation, and signal amplification allows for the detection of low-abundance targets.
To learn more about the SignalStar Multiplex IHC technology, visit the SignalStar Multiplex IHC Application page.
SignalStar Multiplex IHC technology is now available in the US and select European countries, launching globally in October 2023*.
*Check with your local Sales representative for availability in your region.