In this Interview News Med talks to Dr. Nidhi Dey about immune checkpoints IDO1 and PDL1 and how they relate to cutaneous leishmaniasis research.
What are IDO1 and PDL1, and how do they relate to the examination of cutaneous leishmaniasis?
IDO1 and PDL1 function as immune checkpoints, regulating the immune system and potentially impacting the study of cutaneous leishmaniasis. These checkpoints play a role in suppressing T-cell function, a vital aspect of the immune response against the disease-causing parasite.
Their ability to inhibit T-cell activity may impede the body's effectiveness in combating the parasite. Therefore, understanding these immune checkpoints is crucial for developing therapies that target them, aiming to enhance the immune response against the parasite.
In a recent study on cutaneous leishmaniasis patients, it was noted that the transcripts of IDO1 and PDL1 were down-regulated, indicating a reduction in their expression after treatment. This observation is significant as it suggests that treatment may diminish the inhibitory effects of IDO1 and PDL1 on T-cell function.
The down-regulation of IDO1 and PDL1 is a positive outcome, potentially improving the immune system's ability to combat the parasite. This reduction in immune checkpoint expression could lead to a more effective immune response and improved treatment outcomes for patients.
PDL1 serves as a predictive factor for the duration of treatment. Can you explain how this data can influence the care provided to patients?
The finding that PDL1 expression functions as a predictor of treatment duration holds significance for patient care. Individuals exhibiting lower PDL1 expression during treatment displayed quicker responses to therapy.
Utilizing this data allows the evaluation of treatment progress in each patient. Through the monitoring of PDL1 expression, healthcare providers can pinpoint individuals poised for faster recovery.
This information aids in optimizing treatment approaches, potentially shortening treatment duration, lessening patient discomfort, and enhancing overall patient outcomes.
Which methods were employed in the research to examine the spatial arrangement of IDO1 and PDL1 in the skin tissue of individuals affected by cutaneous leishmaniasis?
The research used various methods to examine how IDO1 and PDL1 are distributed in the skin tissue of individuals with cutaneous leishmaniasis. These approaches encompassed low-resolution transcriptome profiling, NanoString DSP, spatial transriptomics using 10x Genomics Visium technology, whole slide imaging and single-cell image analysis with StrataQuest, TissueGnostics.
Employing these techniques enabled investigators to scrutinize the positioning and manifestation of IDO1 and PDL1 in the tissue samples. Through multiplexed imaging and transcriptome profiling, valuable insights were obtained regarding the distribution of these immune checkpoints within the skin lesions.
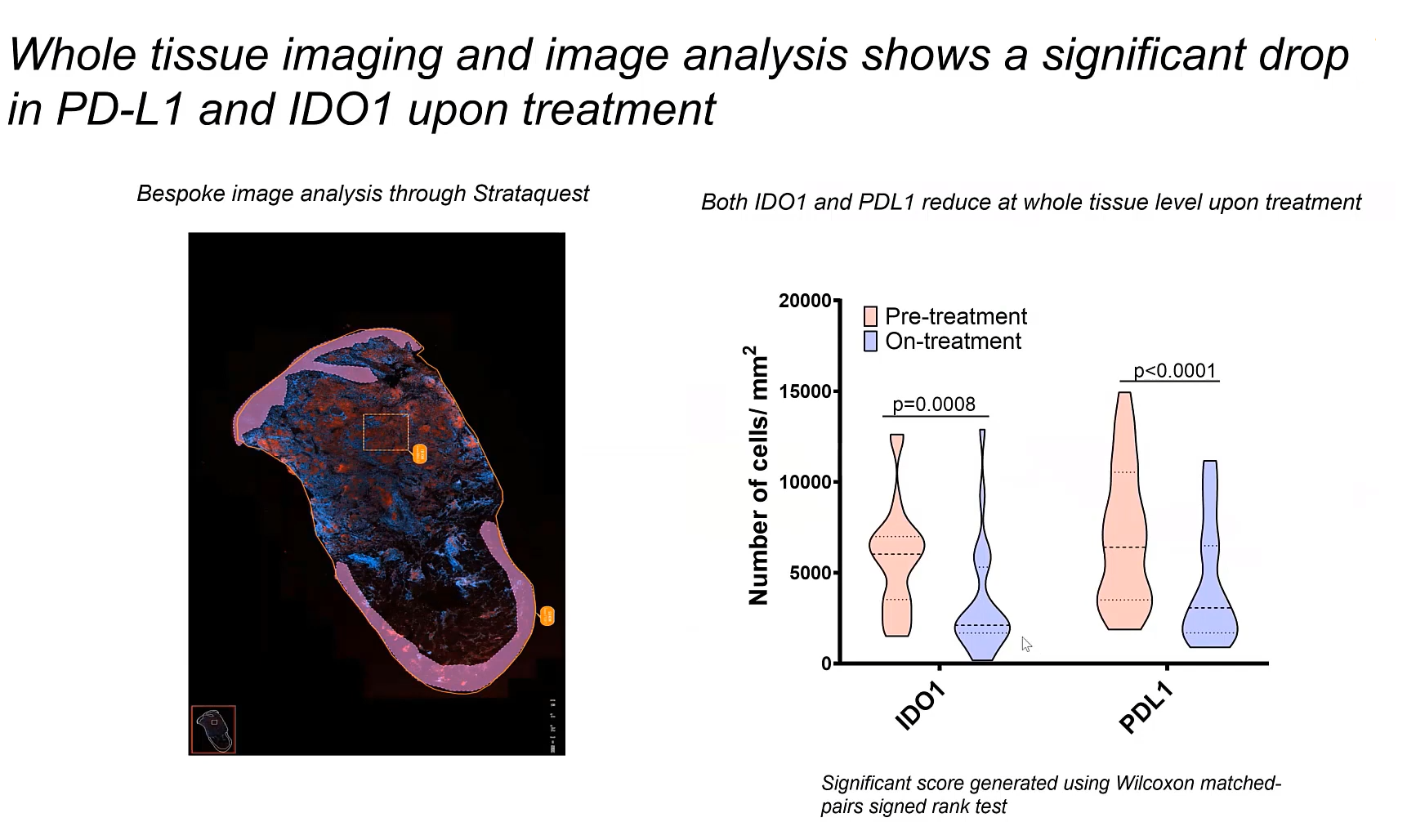
Image Credit: Walrad and Lagos laboratory at the Centre for Immunology and Infection (CII)
How may the interplay of IDO1 with PDL1-positive cells and CD8 memory T cells impact the advancement of the illness and potential approaches to treatment?
During this study, it was found that among top cell types neighboring IDO+PDL1+ cells are CD8 memory T cells which indicates that IDO+PDL1+ cells signal to the neighboring T cells by reducing their T-cell function. Among other cell types regulatory T cells, dendritic cells and type 1 IFN macrophages were detected in the close vicinity.
These immune checkpoints, namely IDO1 and PDL1, have the capacity to hinder the function of CD8 T memory cells, which play a crucial role in eliminating the parasite responsible for causing cutaneous leishmaniasis. If the activity of CD8 memory cells is inhibited, the disease can persist, allowing the parasite to thrive.
Compared to other models of leishmaniasis in India and Brazil, shared genes were found to be associated with IDO1 and PDL1. What do these common genes signify for upcoming research or the advancement of therapies?
Recognizing shared genes linked to IDO1 and PDL1 in diverse leishmaniasis models implies a crucial involvement of these genes in the ailment, regardless of the particular strain.
These common genetic elements carry substantial importance for upcoming research and the creation of treatments.
The recognized shared genes could be focal points for therapy applicable to different leishmaniasis strains. This might accelerate the progress of novel treatments effective against a diverse array of leishmaniasis variations, potentially enhancing therapeutic results and expanding the range of research for this infectious condition.
Can you explain the idea of host-directed treatments concerning cutaneous leishmaniasis and how IDO1 and PDL1 play a role in this strategy?
Host-directed therapies aim to enhance the outcome of infectious diseases by targeting the host's immune response. In the case of cutaneous leishmaniasis, the utilization of IDO1 and PDL1 is fitting as they serve as immune checkpoints, impeding T-cell function.
By directing therapies specifically at these immune checkpoints, such as through immunotherapy or small molecule inhibitors, the strength of the host's immune response against the parasite.
This strategy seeks to diminish the suppression of T-cell function, allowing the immune system to establish a stronger defense against the parasite. Consequently, IDO1 and PDL1 emerge as potential targets for host-directed therapies in treating cutaneous leishmaniasis.
What treatment choices, like immunotherapy or small molecule inhibitors, exist for addressing IDO1 and PDL1 in managing cutaneous leishmaniasis?
In managing cutaneous leishmaniasis, potential approaches for targeting IDO1 and PDL1 include the use of immunotherapy and small molecule inhibitors. FDA-approved PDL1 antibodies are already accessible in the market, representing a type of immunotherapy.
Small molecule inhibitors directed at IDO1 are currently undergoing diverse phases of clinical trials. These treatments aim to counteract the inhibitory impacts of IDO1 and PDL1 on T-cell function, potentially improving the immune response against the parasite responsible for the disease.
Employing these therapeutic choices could result in more efficient and focused strategies for handling cutaneous leishmaniasis.
How did CD68, a myeloid marker, contribute to assessing IDO1 and PDL1 expression in skin tissue, and what is its connection to parasitic burden?
CD68, a myeloid marker, was employed in the investigation to detect macrophages within the skin tissue. These cells were observed to exhibit IDO1 and PDL1. The assessment performed with StrataQuest image analysis software disclosed a direct relationship between the presence of IDO1 and PDL1 and the amastigote burden, indicative of the parasite load.
The presence of IDO1 and PDL1 in macrophages could be associated with the parasite load in cutaneous leishmaniasis. Therefore, these immune checkpoints in myeloid cells may play a role in controlling the parasite burden, potentially limiting the disease's progression.
What could be the implications for clinical practice or the creation of new treatments based on the research results regarding the function of immune checkpoints in cutaneous leishmaniasis?
The research results concerning the immune checkpoints, IDO1 and PDL1, and their role in cutaneous leishmaniasis may significantly influence clinical practices and the creation of novel treatments. From a clinical perspective, utilizing PDL1 as an indicator for treatment duration can optimize treatment plans, lessening the duration and enhancing patient care.
Overall, these insights enhance our understanding of cutaneous leishmaniasis and suggest promising directions for creating more effective treatments, which could improve the management and outcomes of this infectious disease.
About Nidhi Dey
Nidhi Dey is a post-doctoral researcher in the Kaye, Walrad and Lagos laboratory at the Centre for Immunology and Infection (CII). She is currently working on Tegumentary Leishmaniasis using in-situ hybridisations to locate parasites and to identify molecular signatures of host response to the disease using tissue image cytometry and spatial transcriptomics.
About TissueGnostics
TissueGnostics (TG) is an Austrian company focusing on integrated solutions for high content and/or high throughput scanning and analysis of biomedical, veterinary, natural sciences, and technical microscopy samples.
TG has been founded by scientists from the Vienna University Hospital (AKH) in 2003. It is now a globally active company with subsidiaries in the EU, the USA, and China, and customers in 30 countries.
TissueGnostics portfolio
TG scanning systems are currently based on versatile automated microscopy systems with or without image analysis capabilities. We strive to provide cutting-edge technology solutions, such as multispectral imaging and context-based image analysis as well as established features like Z-Stacking and Extended Focus. This is combined with a strong emphasis on automation, ease of use of all solutions, and the production of publication-ready data.
The TG systems offer integrated workflows, i.e. scan and analysis, for digital slides or images of tissue sections, Tissue Microarrays (TMA), cell culture monolayers, smears, and other samples on slides and oversized slides, in Microtiter plates, Petri dishes and specialized sample containers. TG also provides dedicated workflows for FISH, CISH, and other dot structures.
TG analysis software apart from being integrated into full systems is fully standalone capable and supports a wide variety of scanner image formats as well as digital images taken with any microscope.