Obesity, a pervasive public health issue, demands effective and safe therapeutics. Incretin-based treatments, particularly glucagon-like peptide 1 receptor (GLP-1R) agonists, have demonstrated significant weight reduction and cardiovascular benefits. However, the demand persists for obesity treatments with enhanced efficacy, less frequent dosing, and improved tolerability. To address this need, researchers explored the use of multi-specific agonist peptides against GLP-1 and gastric inhibitory peptide (GIP) pathways.
Genome-wide studies supported the contribution of the GIP receptor (GIPR) locus to body weight regulation. Bispecific molecules engineered by combining GIPR antagonism with GLP-1R agonism showed promising preclinical results, inducing weight loss and metabolic improvements in obese mice and monkeys. AMG 133 is one such bispecific molecule developed by conjugating a human monoclonal GIPR-antibody with two GLP-1 analog agonist peptides. Researchers in the present study investigated the safety, pharmacological properties, and efficacy of AMG 133 in preclinical and clinical settings.
About the study
In the present study, AMG 133 was synthesized as an antibody-peptide conjugate using an amino acid linker, and its pharmacokinetic (PK) properties were characterized in vitro. The cell-based functional assays involved the use of recombinant human embryonic kidney (HEK) 293T cells expressing human or cynomolgus monkey GIPR and Chinese hamster ovary (CHO) cells expressing rat or mouse GIPR. Cyclic adenosine monophosphate (cAMP) accumulation was measured. The PK properties of intact and total AMG 133 were evaluated by injecting the molecule in mice and obese female cynomolgus monkeys.
In the clinical segment of the study, a phase 1, randomized, placebo-controlled, double-blind trial was conducted to evaluate the tolerability, safety, PK, and pharmacodynamics (PD) of single ascending doses (SADs) and multiple ascending doses (MADs) of AMG 133 in adults with obesity. While the primary endpoints were safety and tolerability, the secondary endpoints were PK and immunogenicity. Further, PD biomarkers (including weight) were considered as exploratory endpoints.
In seven SAD cohorts, 49 obese participants were enrolled and randomized to receive AMG 133 (21–840 mg) or placebo for up to 150 days. The mean age of these participants was 45.5 to 53.8 years and their body mass index (BMI) was 32.5 to 34.8 kg m−2 In three MAD cohorts, 26 obese participants were enrolled and randomized to receive AMG 133 (140, 280 or 420 mg) or placebo for up to 207 days. The mean age of these participants was 40.3 to 51.6 years and their BMI was 32.5 to 34.2 kg m−2. None of the participants had a history of diabetes mellitus.
Results and discussion
The molecular weight of AMG 133 was found to be 153,514 Da. In the cellular assays, AMG 133 showed antagonist activity against human, cynomolgus monkey, and rat GIPR. The AMG 133 murine surrogate could reduce food intake and blood glucose levels and induce weight loss in mice. Dose-dependent improvements in blood glucose, plasma insulin, and lipid levels were also observed. AMG 133 treatment in obese monkeys resulted in a reduction in body weight, total energy intake, fasting triglycerides, insulin, and cholesterol after six weeks.
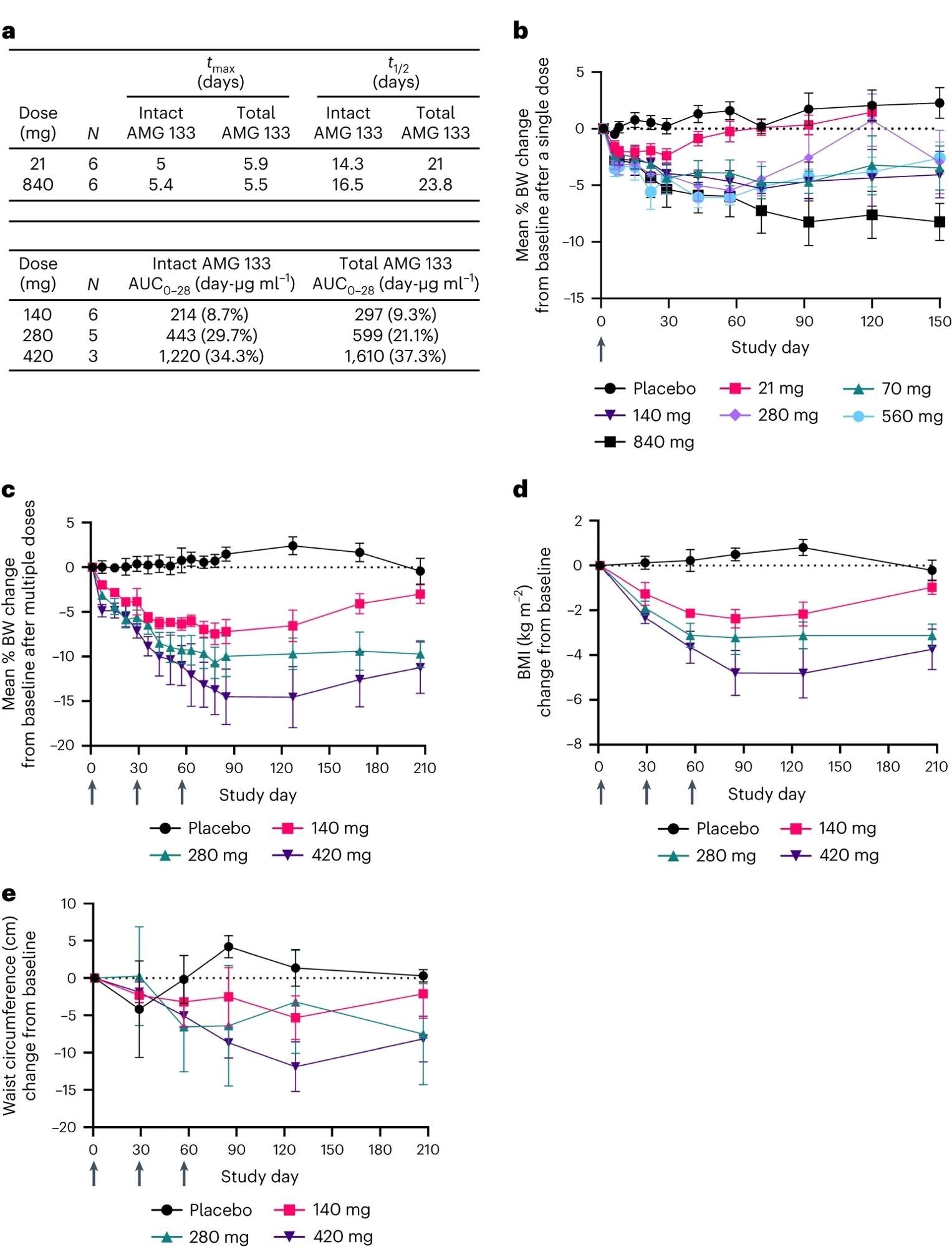 a, AMG 133 PK parameters; tmax is presented as the median and t1/2 is presented as the geometric mean. AUC0–28 is presented as the geometric mean (CV%) after the last dose of AMG 133 on day 57 for MAD cohorts. b–e, Mean (s.e.m.) percent change from baseline in BW after single doses, n = 6–7 for AMG 133 and n = 12 for placebo at day 1 (b) and multiple doses, n = 6–8 for AMG 133 and n = 6 for placebo at day 1 (c). Mean (s.e.m.) change from baseline in BMI, n = 6–8 for AMG 133 and n = 6 for placebo at day 1 (d) and waist circumference, n = 6–8 for AMG 133 and n = 6 for placebo at day 1 (e) after multiple doses of AMG 133. Arrows indicate when the investigational product was administered: at day 1 in the SAD cohorts (b) and at day 1, 29 and 57 in the MAD cohorts (c–e).
a, AMG 133 PK parameters; tmax is presented as the median and t1/2 is presented as the geometric mean. AUC0–28 is presented as the geometric mean (CV%) after the last dose of AMG 133 on day 57 for MAD cohorts. b–e, Mean (s.e.m.) percent change from baseline in BW after single doses, n = 6–7 for AMG 133 and n = 12 for placebo at day 1 (b) and multiple doses, n = 6–8 for AMG 133 and n = 6 for placebo at day 1 (c). Mean (s.e.m.) change from baseline in BMI, n = 6–8 for AMG 133 and n = 6 for placebo at day 1 (d) and waist circumference, n = 6–8 for AMG 133 and n = 6 for placebo at day 1 (e) after multiple doses of AMG 133. Arrows indicate when the investigational product was administered: at day 1 in the SAD cohorts (b) and at day 1, 29 and 57 in the MAD cohorts (c–e).
In the phase 1 clinical study, AMG 133 showed an acceptable safety and tolerability profile. Clinical safety laboratory parameters (electrolytes, kidney function, and hematology) and echocardiogram parameters showed no significant differences between the treatment groups. No severe or serious adverse events (AEs) were reported. The common AEs were mild gastrointestinal symptoms, predominantly nausea and vomiting, which generally resolved within 48 hours. Although a reduction was observed in fasting glucose levels, no hypoglycemia-related events were reported.
Furthermore, no clinically significant changes in blood pressure were observed with AMG 133, and heart rate increases within the normal range were noted. AMG 133 treatment led to increased free fatty acids, particularly in the 420 mg group. Transient decreases in total cholesterol, low-density lipoprotein, and triglycerides were observed across groups (including placebo).
AMG 133 showed a dose-proportional increase with maximum plasma concentrations attained around 4 to 7 days post-dose in the SAD cohort and after 4 to 6 days in the MAD cohort. The mean half-life for intact AMG 133 ranged from 14 to 16 days, and that for total AMG 133 ranged from 21 to 24 days.
Importantly, AMG 133 treatment was found to lower the mean body weight, BMI, and waist circumference from baseline in a dose-dependent manner in the participants.
Conclusion
In conclusion, this study's findings suggest that AMG 133 may be a potentially viable therapeutic option for weight management, given its favorable safety profile, extended half-life, and substantial and sustained weight loss. Further research in a phase 2 clinical trial setting is warranted to confirm these findings.
Journal reference:
- A GIPR antagonist conjugated to GLP-1 analogues promotes weight loss with improved metabolic parameters in preclinical and phase 1 settings. Véniant, M.M. et al., Nature Metabolism (2024), DOI: 10.1038/s42255-023-00966-w, https://www.nature.com/articles/s42255-023-00966-w