Breast cancer is a highly heterogeneous disease with phenotypically and genetically diverse cell groups. The most common risk factors for the development of this disease include gender, ethnicity, genetic makeup, lifestyle, geographical factors, and radiation exposure.
Breast cancer is the second leading cause of cancer-related death among women in the United States. About 13% of US women are likely to develop breast cancer in their lifetime.
Among various subtypes, triple-negative breast cancer (TNBC) is an aggressive phenotype accounting for about 10 – 15% of all breast cancer cases. The absence of estrogen, progesterone, and human epidermal growth receptors in TNBC makes it challenging to treat with standardized hormone therapy.
Kaempferol metabolism
Studies investigating the therapeutic potential of phytochemicals have identified several natural compounds with anticancer and chemopreventive properties, including taxanes, vinblastine, vincristine, and podophyllotoxin analogs. These compounds have also been found to mitigate chemoresistance and increase the chemo-sensitivity of cancer cells toward standard chemotherapeutic agents.
Kaempferol is a natural flavonoid with antioxidant, anti-inflammatory, anticancer, chemopreventive, cardioprotective, and neuroprotective properties. It is commonly found in vegetables like broccoli, capers, cabbage, onion, green peas, kale, and spinach, and in berries like strawberries, gooseberries, and blackberries.
This lipophilic compound is absorbed mainly through the small intestine and extensively metabolized in the liver to generate methyl or sulfate salts. The intestinal microbiota also metabolizes kaempferol into aglycones and some phenolic compounds.
All metabolites of kaempferol are absorbed into the systemic circulation, distributed in various tissues, and excreted in feces and urine.
Kaempferol in cancer management
Anti-tumorigenic, anti-proliferative, and pro-apoptotic activities of kaempferol have been observed against various cancer types, including liver, lung, colorectal, pancreatic, kidney, bone, ovarian, and breast cancers.
Kaempferol, used in combination with chemotherapeutic agents, has been found to increase the efficacy of chemotherapy by chemo-sensitizing cancer cells. Kaempferol has also been found to reverse the chemoresistance of standard chemotherapeutic agents by modulating various intracellular signaling pathways.
Anticancer mode of action of kaempferol in breast cancer
Lifelong exposure to endogenous and exogenous estrogen is known to be associated with the risk of breast cancer development. Despite being the most widely consumed dietary phytoestrogen, kaempferol has been found to exert anticancer activity in both estrogen-dependent and estrogen-independent manners.
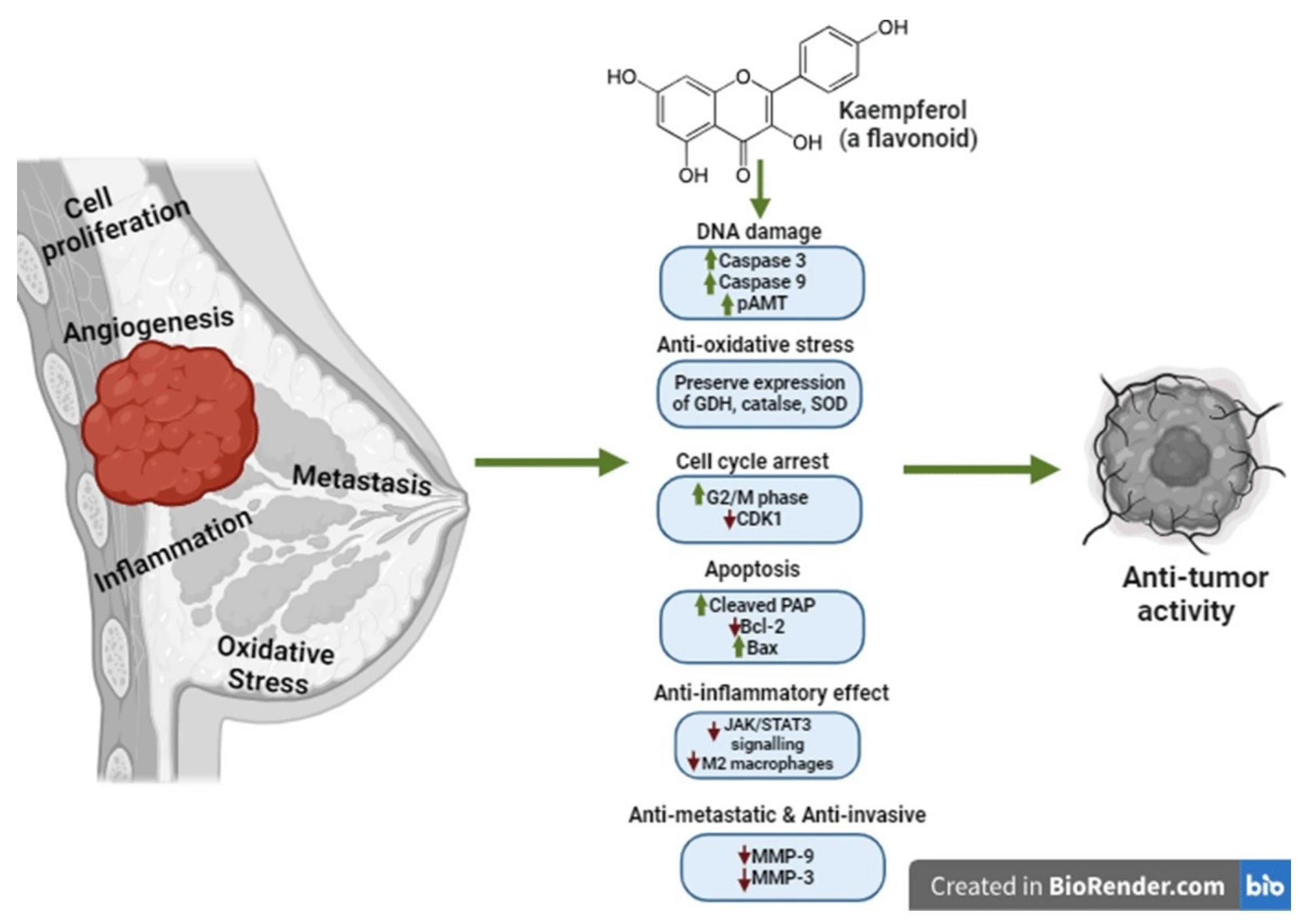 The effect of the flavonoid kaempferol on the development and progression of breast cancer. Green arrows indicate induction and red arrows indicate inhibition.
The effect of the flavonoid kaempferol on the development and progression of breast cancer. Green arrows indicate induction and red arrows indicate inhibition.
In both estrogen-dependent and estrogen-independent breast cancer cells, kaempferol has been found to increase DNA synthesis at low concentrations and inhibit DNA synthesis and cell growth at high concentrations.
Kaempferol has been found to exert both estrogenic and anti-estrogenic activities depending on the concentration used. Its estrogen agonistic activity is known to be associated with inhibition of DNA synthesis and cell growth.
Available evidence indicates that kaempferol inhibits the proliferation of TNBC cells by inducing apoptosis and cell cycle arrest at the G2/M phase. It can also inhibit the fatty acid synthetase enzyme to reduce cell growth and induce apoptosis in breast cancer cells overexpressing this enzyme.
Evidence collected from 2D and 3D cell culture models indicates that kaempferol induces breast cancer cell apoptosis by modulating the ERK/MEK1/ELK1 signaling pathway.
Regarding targeting estrogenic pathways, evidence indicates that kaempferol antagonizes estrogen-mediated breast cancer cell proliferation by upregulating the expression of cathepsin, cyclin D1, and cyclin E and downregulating the expression of Bax and p21.
Furthermore, kaempferol has been found to prevent the malignant transformation of breast cancer cells by inhibiting cell proliferation via estrogen-dependent pathways.
Kaempferol, at low and high concentrations, has been found to reduce the viability of estrogen-positive and estrogen-negative breast cancer cells, respectively. These effects are associated with reduced mRNA and protein expressions of estrogen-alpha and reduced expressions of progesterone receptor, insulin receptor, and cyclin D1.
Regarding antioxidant activity, evidence indicates that kaempferol inhibits the formation of neutrophil extracellular traps, reducing the production of reactive oxygen species (ROS) and inhibiting breast cancer metastasis.
Kaempferol has been found to synergize the anti-oxidative activity of fisetin (another anticancer agent) and activate gamma-H2A histone family member X (H2AX), leading to DNA damage and apoptosis.
Doxorubicin, a well-established chemotherapeutic agent, is known to induce various adversities, including vascular toxicity and cardiotoxicity. Kaempferol has been found to reverse these adversities by regulating ROS levels.
Regarding anti-metastatic and anti-invasive activities, evidence indicates that kaempferol inhibits the activation of RhoA, Rac, and GTP-binding proteins involved in microfilament arrangement, leading to inhibition of TNBC cell migration.
Kaempferol has also been found to inhibit TNBC cell adhesion, motility, and migration by reducing the expression of matrix metalloproteinase-2 (MMP-2) and MMP-9.
Pharmaceutical formulations of kaempferol
Several formulations and delivery systems have been developed to overcome the challenges associated with poor water solubility and limited bioavailability of kaempferol.
Kaempferol-loaded nanoemulsion MNE has been developed with chitosan for muco-adhesion to achieve nasal delivery. The product has shown high permeability across the nasal mucosa and high anticancer activity against glioma cells.
Kaempferol gold nanoparticles have also shown robust biocompatibility and increased pro-apoptotic and anti-angiogenic activities in breast cancer cells.
Clinical evidence
A clinical trial involving healthy adults has shown that a daily dose of high-dose kaempferol for four weeks is not associated with adverse events.
Another trial involving male smokers has shown that high-level dietary intake of kaempferol is associated with a reduction in pro-inflammatory mediators.
More clinical trials are needed to understand the potential of kaempferol in cancer management conclusively.