Study findings highlighted that esaxerenone met or exceeded trichlormethiazide's efficacy, as demonstrated in the former's morning home blood pressure (BP)-reducing effects. As with previous clinical trials, esaxerenone's safety was comparable to trichlormethiazide's, highlighting the former's potential as a replacement or alternative.
 Study: Home blood pressure-lowering effect of a non-steroidal mineralocorticoid receptor blocker, esaxerenone, versus trichlormethiazide for uncontrolled hypertension: the EXCITE-HT randomized controlled study. Image Credit: Adheamir / Shutterstock
Study: Home blood pressure-lowering effect of a non-steroidal mineralocorticoid receptor blocker, esaxerenone, versus trichlormethiazide for uncontrolled hypertension: the EXCITE-HT randomized controlled study. Image Credit: Adheamir / Shutterstock
Background
Hypertension is a medical condition characterized by excessively high blood pressure in a patient's blood vessels (usually 140/90 mmHg or higher). It is a common condition, with the World Health Organization (WHO) estimating its global prevalence at more than 30% of the adult population (1.28 billion individuals). Given its association with a heightened risk of potentially lethal comorbidities, including cardiovascular disease (CVD) and cerebrovascular conditions, timely and efficient treatment of the condition is a public health priority.
Fortunately, decades of research have resulted in the discovery of numerous antihypertensive agents. Unfortunately, there remains a disparity in specific populations' responses to these treatments. While Korea and Taiwan have achieved adequate blood pressure (BP) control in ~70% of the population, Japan has only achieved the same for ~35% of its citizens.
This disparity has been termed the 'hypertension paradox.' Current research suggests that this paradox arises from patients' varied responses to single-agent interventions, necessitating combinations of antihypertensive agents or novel drug discovery.
Esaxerenone is a next-generation antihypertensive drug belonging to the non-steroidal mineralocorticoid receptor blocker (MRB) family. Previous clinical studies have suggested its higher selectivity, longer half-life, improved potency, and superior bioavailability than other MRB drugs. Unfortunately, due to a lack of clinical evidence from Japan, its use in the Japanese population remains lacking.
About the study
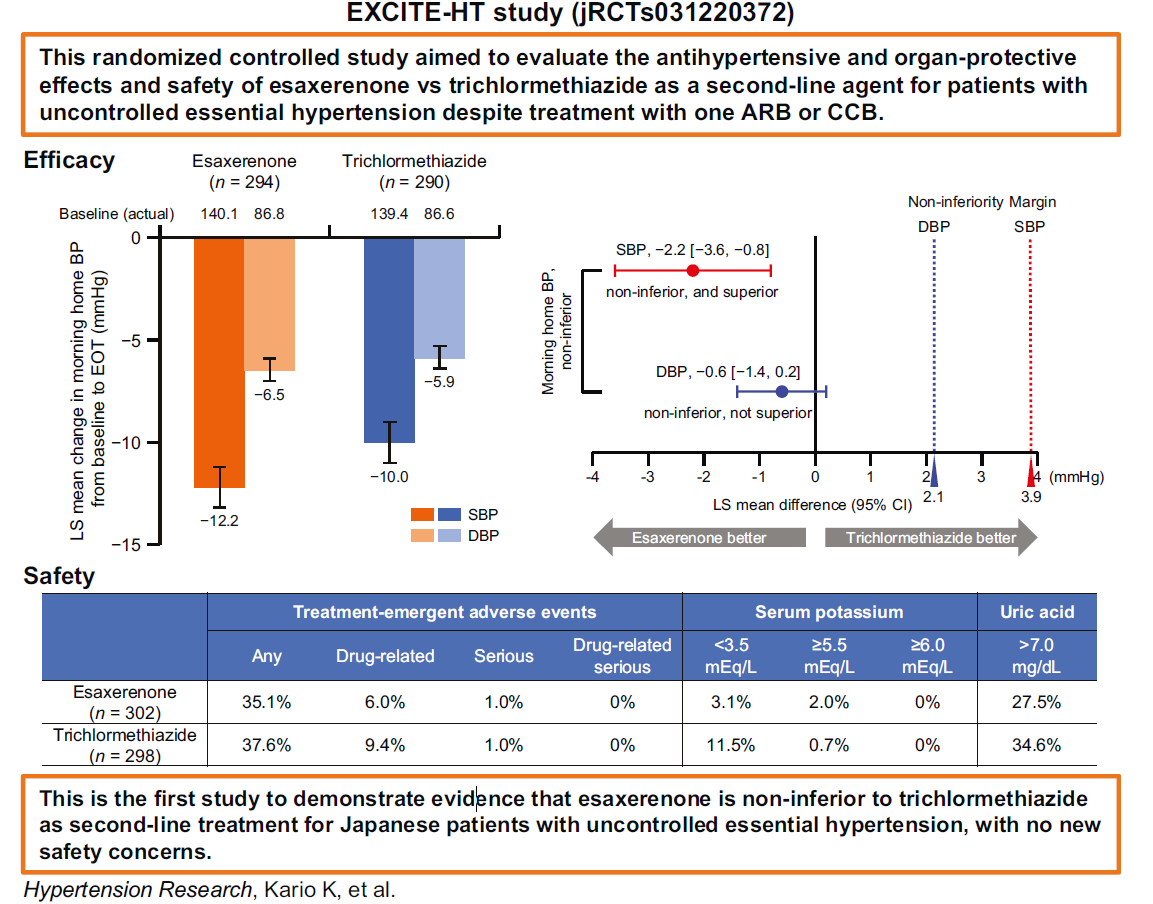
The present study, termed 'EXCITE-HT,' aimed to compare the antihypertensive efficacy and safety of esaxerenone versus trichlormethiazide, the current second-line agent choice against uncontrolled essential hypertension. The randomized controlled study was conducted across 54 centers between December 2022 and September 2023.
The study cohort comprised participants above 20 years who presented persistently high (systolic BP [SBP] ≥ 125 mmHg or diastolic BP [DBP] ≥ 75 mmHg) home BPs despite a minimum of four weeks of single-agent angiotensin II receptor blockers (ARBs) or calcium channel blockers (CCBs). Participants meeting inclusion criteria were randomly assigned to the esaxerenone or trichlormethiazide cohort.
Study interventions consisted of a four-week-long run-in period, following which the esaxerenone cohort was provided with 2.5 mg/day or 1.5 mg/day of the drug (depending on the estimated glomerular filtration rate [eGFR] or the presence of preexisting chronic disease) in addition to their regular ARB or CCB dose. Correspondingly, the trichlormethiazide cohort was provided a 1 mg/day dose of the drug in combination with their preexisting antihypertension medication.
Response to the respective drugs was evaluated via changes in home BP values recorded twice daily (morning and bedtime). Measurements were made using an upper arm cuff sphygmomanometer. Primary study endpoints were calculated by computing the difference between baseline BP records and those at the end of treatment (EOT; after 12 weeks). Additionally, changes in serum N-terminal pro-brain natriuretic peptide (NT-proBNP) and urinary albumin-to-creatinine ratio (UACR) were computed as secondary endpoints.
Drug safety was measured using laboratory test values of eGFR, electrolytes, uric acid (UA), and treatment-emergent adverse events (TEAEs).
Study findings
Of the 736 assessed for eligibility, 600 met the study inclusion criteria and were enrolled. Of these, five discontinued the study, resulting in 295 patients in the esaxerenone cohort and 290 in the trichlormethiazide cohort. Between-group demographics and BP analyses revealed that cohorts were statistically indistinguishable at baseline.
BP reduction analyses revealed that esaxerenone and trichlormethiazide reduced the mean morning SBP by 12.2 mmHg and 10.0 mmHg, respectively. Morning DBP was similarly reduced by 6.5 mmHg and 5.9 mmHg. While esaxerenone outperformed trichlormethiazide in both measures, morning DBP reductions were statistically (and clinically) indistinguishable. However, morning SBP reductions highlight the potential for esaxerenone to reduce CVD risk by between 3.5 and 9.5% more than similar doses of trichlormethiazide.
Safety evaluations revealed similar safety levels between both drugs – TEAEs were observed in 35.1% of esaxerenone and 37.6% of trichlormethiazide patients, respectively. Of these, only 6.0% and 9.4% were drug-associated. Most TEAEs were mild to moderate, with serious TEAEs (n = 6; 3 per cohort) found to be non-study drug-related.
Conclusions
The EXCITE-HT trial is the first to evaluate the efficacy and safety of esaxerenone as a combination anti-hypertension drug and its first clinical trial on a Japanese population. Study findings revealed that the next-generation hypertension intervention was non-inferior to trichlormethiazide across both efficacy and safety. These findings suggest that esaxerenone can replace or serve as an alternative to trichlormethiazide in Japanese hypertension patients.
Journal reference:
- Kario, K., Ohbayashi, H., Hashimoto, M., Itabashi, N., Kato, M., Uchiyama, K., Hirano, K., Nakamura, N., Miyamoto, T., Nagashima, H., Kajiyama, S., Ishida, H., Imai, E., Ebe, Y., Ohishi, M., Katsuya, T., Taguchi, T., Tanabe, A., & Shimosawa, T. (2024). Home blood pressure-lowering effect of a non-steroidal mineralocorticoid receptor blocker, esaxerenone, versus trichlormethiazide for uncontrolled hypertension: The EXCITE-HT randomized controlled study. Hypertension Research, 1-12, DOI – 10.1038/s41440-024-01762-z, https://www.nature.com/articles/s41440-024-01762-z