Background
The regulation of the hunger-satiety cycle involves complex interactions between central and peripheral signals, primarily integrated in the hypothalamus, especially the arcuate nucleus. Two neuronal populations in the arcuate nucleus- Neuropeptide Y (NPY)/Agouti-related protein (AgRP) (orexigenic) and pro-opiomelanocortin (POMC)/cocaine- and amphetamine-regulated transcript (CART) (anorexigenic)-respond to signals from the adipose tissue, pancreas, and gut, influencing food intake and energy balance. Gut hormones like GLP-1, Cholecystokinin (CCK), and Peptide YY (PYY) affect both central and peripheral mechanisms, including gastric motor activity, to regulate satiety.
The central vs. peripheral effects of GLP-2 play a significant role in its overall impact on food intake. While GLP-2's central anorexigenic effects are primarily mediated through its action on the hypothalamic melanocortin pathway, its peripheral effects on gastric motility and nutrient absorption further influence satiety. Understanding this dual mechanism is crucial for developing therapeutic interventions.
Further research is needed to fully understand the mechanisms by which GLP-2 influences gastrointestinal motor activity and its potential role in regulating food intake, which could lead to new therapeutic approaches for obesity.
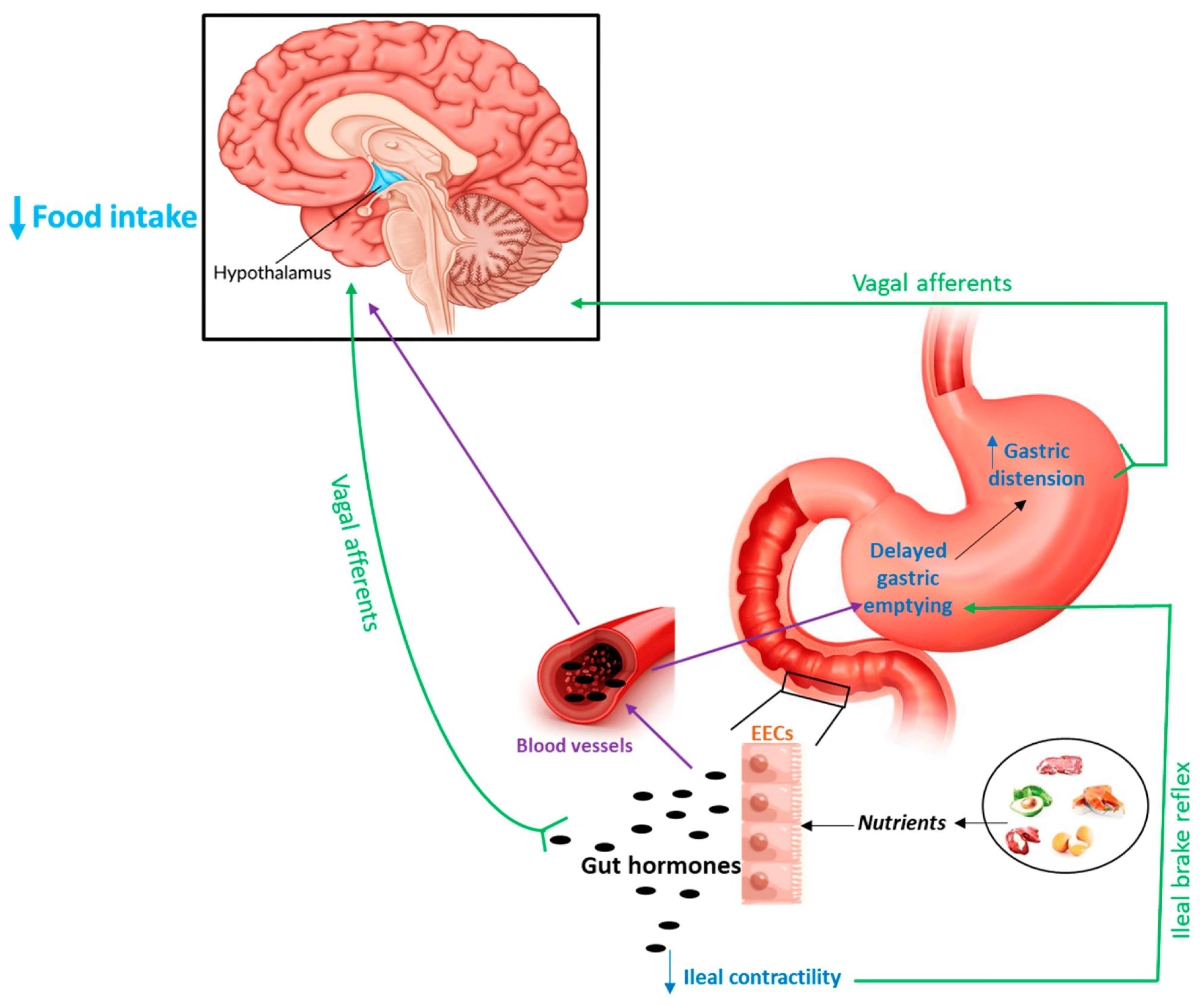 Schematic representation of the main mechanisms through which gut hormones may influence hypothalamic structures to induce anorexigenic effects. Purple lines (bloodstream); green lines (nervous fibers); and EECs (enteroendocrine cells).
Schematic representation of the main mechanisms through which gut hormones may influence hypothalamic structures to induce anorexigenic effects. Purple lines (bloodstream); green lines (nervous fibers); and EECs (enteroendocrine cells).
Gut-brain axis and satiety signals
Hormones derived from the gut can influence food intake through various mechanisms. One prominent route involves the vagal nerve, where receptors located in the gut mucosa activate vagal afferent fibers. These signals travel to the hypothalamus through the nucleus tractus solitarius (NTS). Hormones like GLP-1, CCK, and PYY are well-known for their effects on gastrointestinal motility and the subsequent generation of satiety signals. Gastric emptying and gastric accommodation are two key processes involved in these signals. Slower gastric emptying, for example, increase satiety by prolonging the presence of food in the stomach, a feature commonly seen in obese individuals with faster emptying rates.
In contrast, GLP-2’s effects on food intake are modulated both centrally, through the activation of the melanocortin receptor-4 (MC4-R), and peripherally, by delaying gastric emptying via vagal afferent pathways. This dual modulation suggests that GLP-2 might act as a bidirectional regulator within the gut-brain axis, influencing food intake through both central and peripheral mechanisms.
GLP-2: origin and function
GLP-2 is a 33-amino acid peptide primarily produced in the intestines by L-cells (gut cells that release hormones to regulate digestion and appetite) and is also expressed in the brain. Both GLP-1 and GLP-2 are derived from the proglucagon protein. While GLP-1 is better known for its role in promoting satiety and enhancing insulin secretion, GLP-2's role in modulating gastrointestinal motility and promoting intestinal growth is also significant. Unlike GLP-1, GLP-2's half-life is brief due to rapid degradation by the enzyme dipeptidyl peptidase IV (DPP-IV). Thus, DPP-IV-resistant analogs of GLP-2 have been developed for therapeutic uses, especially in treating short bowel syndrome (SBS).
The interplay between GLP-2 and the gut microbiota is another emerging area of interest. Recent research suggests that gut microbiota can influence the secretion of GLP-2, thereby indirectly affecting food intake. This relationship underscores the potential for microbiota-targeted therapies to modulate GLP-2 levels and, consequently, regulate appetite and energy balance.
GLP-2 and gastrointestinal motility
GLP-2 has been shown to impact the motility of the gastrointestinal tract. In animal studies, GLP-2 administration resulted in delayed gastric emptying and reduced antral motility, which is believed to contribute to increased satiety and reduced food intake. This effect is likely mediated by the central nervous system, particularly through the activation of melanocortin receptor-4 (MC4-R). Additionally, the vagus nerve may play a role, as GLP-2 receptors have been found on vagal afferent neurons, suggesting a pathway through which GLP-2 signals can affect food intake regulation.
Conflicting findings in GLP-2 research
Despite these promising findings, research on GLP-2 has produced conflicting results, particularly in humans. While some animal studies demonstrate significant appetite suppression and delayed gastric emptying following GLP-2 administration, similar effects have not been consistently observed in human trials. Peripheral administration of GLP-2 in healthy individuals has shown no significant impact on appetite or postprandial satiety, highlighting the need for further investigation into the mechanisms of action and potential therapeutic applications of GLP-2 in human populations.
GLP-2 and the enteric nervous system
GLP-2 also affects the enteric nervous system by modulating inhibitory and excitatory neurotransmission. The peptide induces gastric relaxation by enhancing the release of nitric oxide (NO) and vasoactive intestinal peptide (VIP), two major inhibitory neurotransmitters. These neurotransmitters cause smooth muscle relaxation in the gastrointestinal tract, slowing motility and contributing to delayed gastric emptying. This could explain why GLP-2, despite promoting nutrient absorption, can still play a role in reducing food intake by extending gastric transit times.
The role of gut microbiota in GLP-2 function
In addition to its direct effects on gastrointestinal motility, GLP-2’s interaction with gut microbiota plays a crucial role in its overall impact on food intake and energy homeostasis. Alterations in gut microbiota composition have been shown to affect GLP-2 secretion, thereby influencing the release of anorexigenic hormones and modifying satiety signals. This relationship suggests that targeting gut microbiota could be a viable strategy for enhancing GLP-2’s therapeutic potential, particularly in managing obesity and related metabolic disorders.
The dual role of GLP-2 in nutrient absorption and satiety
One exciting aspect of GLP-2 is its dual role in enhancing nutrient absorption and promoting satiety. On the one hand, GLP-2 supports the growth and repair of the intestinal mucosa and facilitates nutrient absorption, particularly of glucose, fatty acids, and amino acids. On the other hand, it suppresses food intake by slowing gastric emptying and increasing satiety. These two roles may seem contradictory, but the modulation of satiety signals and the prolonged digestion time could offer a balanced mechanism in energy homeostasis.
GLP-2's therapeutic potential
The role of GLP-2 in regulating food intake and gastrointestinal motility presents a potential therapeutic target for conditions like obesity. While GLP-1 analogs are already used in obesity treatment, GLP-2's ability to influence gut-brain signaling and delay gastric emptying could complement these therapies. In preclinical studies, GLP-2 has been shown to suppress appetite and delay gastric emptying in animals, but similar effects have not been consistently demonstrated in humans.
Conclusion
GLP-2 plays a multifaceted role in regulating food intake through its effects on gastrointestinal motility and the gut-brain axis. GLP-2 could contribute to short-term and long-term food intake control by modulating gastric emptying and influencing satiety signals. However, the conflicting results observed in human studies highlight the need for more research to fully understand its potential therapeutic applications. Its therapeutic potential for obesity and related metabolic disorders warrants further investigation. The peptide's dual role in promoting nutrient absorption and controlling satiety makes it a promising candidate for future treatments targeting metabolic health. The complex interplay between GLP-2, the gut-brain axis, and gut microbiota adds another layer of interest to its potential therapeutic applications.
Journal reference:
- Baccari MC, Vannucchi MG, Idrizaj E. The Possible Involvement of Glucagon-like Peptide-2 in the Regulation of Food Intake through the Gut–Brain Axis. Nutrients. (2024), DOI- https://doi.org/10.3390/nu16183069, https://www.mdpi.com/2072-6643/16/18/3069