Watch Today!
Visualizing the Intracellular Immune Response to SARS-CoV-2 with Fast, High-Content Imaging
The substantial, real-world impact of SARS-CoV-2 inflammatory disease (COVID-19) is intertwined with the immune response occurring inside of individual cells. The innate immune response is the first line of defense that can stop or slow many types of infection. It is critical in mounting systemic antiviral responses and signaling to white blood cells (macrophages). SARS-CoV-2 mediated delays of innate immune response activation in airway epithelium cells might provide an opening for the virus to establish itself and shape the outcome of an infection.
Preservation of the in-situ cellular environment was vital to investigating the interplay between the antiviral and inflammatory roles of the innate immune response to SARS-CoV-2 infection. Automated, high-content imaging (HCI) readily achieves this need and enables direct visualization of the intracellular immune response.
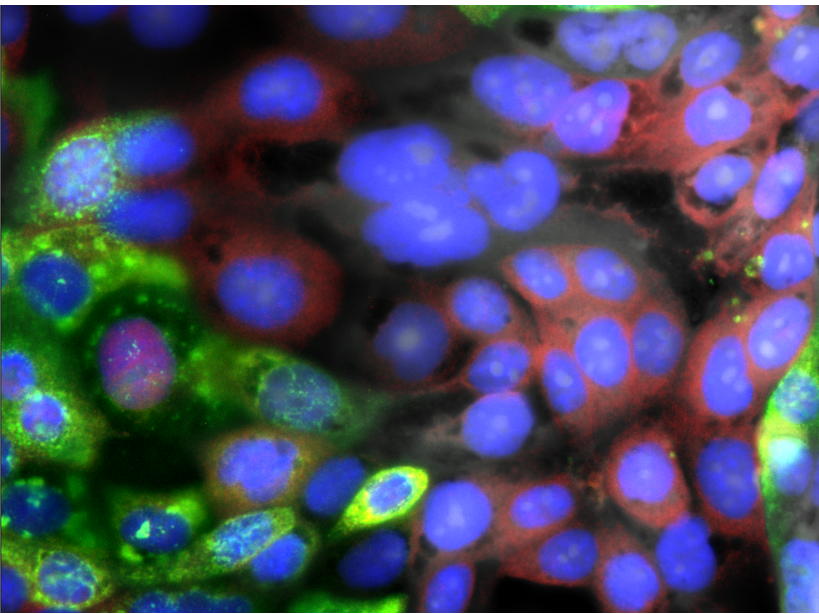
Human airway cells; with SARS-CoV-2 in green, innate immune response in red, and nucleus in blue
About this webinar
In this webinar, Dr. Jason Otterstrom and Dr. Matthew Whelan describe how automated microscopy and image analysis can quantify critical markers of infection.
Dr. Ann-Kathrin Reuschl, co-lead author of two SARS-CoV-2 infection and inflammation studies, describes the insight provided by microscopy and how it relates to complementary techniques. Her results reveal that some SARS-CoV-2 variants have evolved the ability to antagonize the intracellular immune response, providing insight into the emergence of subtypes such as the Alpha and Omicron. Understanding interactions between the virus and the host innate immune response provides a window of opportunity to discover potential therapeutics against severe COVID-19.
Watch a preview of the webinar below.
SARS-CoV-2 Replicates Rapidly in Calu-3 Lung Epithelial Cells
Key learning objectives:
- What is high-content imaging (HCI) and how researchers can use it in 2D cell culture and virology?
- How HCI data and insights integrate into larger -omics studies
- Future directions for HCI in virology to study mechanisms of viral propagation among cells
About the event speakers

Dr. Whelan carried out a Batchelor of Science in Cell and Molecular Biology at University College Dublin, focusing on quantitative microscopy of membrane trafficking and perturbation of Golgi morphology in mammalian cells. Upon graduating in 2015, he was awarded the Government of Ireland PhD Research Scholarship (IRC), allowing him to undertake a PhD project of his own design. This student led award afforded him the opportunity to shift his research focus toward investigating the relationship between intracellular microbial pathogens and their hosts. During his PhD, he applied High Content Screening (HCS) microscopy approaches to investigate the effect of DNA supercoiling mediated epigenetic regulation on biofilm formation and host-cell entry by the pathogen Campylobacter jejuni.
In 2020, he joined the Division of Infection and Immunity (I&I) in UCL as a Research Fellow in ‘HCS Microscopy and Image Analysis’. Under the supervision of Prof. Madhad Noursadeghi, he developed the ‘Host-Pathogen Microscopy Core’ facility, with the primary goal of providing cutting edge HCS and super-resolution microscopy and quantitation of host cell responses to viral and bacterial infections. He is also currently a postdoctoral researcher in the Laboratory of Prof. Clare Jolly in I&I. His own research focuses on the application of live cell and super-resolution approaches to investigate the kinetics of HIV nuclear entry in primary T Cells.

Dr. Otterstrom obtained a Bachelor of Arts in Applied Physics at the University of Utah, during which time he tried his hand at research in fields of comparative physiology, bioengineering, NMR and radiotherapy. As a PhD student at Harvard University, he studied the biophysics of membrane fusion as mediated by influenza virus hemagglutinin protein using single-molecule microscopy techniques. He went on to obtain a Marie Curie fellowship to utilize super-resolution imaging to study chromatin fine-packing structure at the Institute of Photonic Sciences (ICFO) near Barcelona, Spain.
As an application scientist for IDEA Bio-Medical he supports clients with adapting their diverse experiments and assays to be performed in the context of automated microscopy on the company’s flagship product, the WiScan Hermes.

Dr. Ann-Kathrin Reuschl is a postdoctoral Research Associate within the Division of Infection & Immunity at University College London. With a background in infection immunology, she undertook a PhD at Imperial College London during which she explored early interactions between M. tuberculosis and primary human airway epithelium, showing an underappreciated contribution of the epithelium to local inflammation during infection. In 2016, Ann-Kathrin joined Prof Clare Jolly’s team at UCL where she started to interrogate how HIV-1 reprograms its target, the human primary CD4+ T cell.
When the pandemic struck, her research pivoted to SARS-CoV-2 host-pathogen interactions. Ann-Kathrin could draw upon her expertise in airway epithelial cell and infection biology to aid the establishment of a SARS-CoV-2 research program at the Division of Infection and Immunity at UCL. As part of a national and international collaboration, she has contributed to fundamental discoveries about SARS-CoV-2 infection biology as well as exploring therapeutic targets. Crucially, this work showed how SARS-CoV-2 evolution allowed the virus to evade the human innate immune response (Thorne et al, Nature, 2021). This work has implications for the molecular host-pathogen interactions of emerging variants that shape the immunological niche in which the virus resides.
Who should attend this webinar
- Virologists and immunologists looking to improve their throughput and data quantification using HCI
- Life-science researchers who want a picture of what is happening in situ with their samples to measure spatial and morphological phenomena
- People who want to learn how events taking place inside our cells can affect our whole body when we get sick