The current generation of medical devices utilized by therapists, surgeons, doctors, and patients themselves definitely looks a lot more different than how they did just a short while ago.
Standard medical devices were often cumbersome, crude, and in certain cases, not user-friendly. Conversely, just like a majority of industries, the need for connected, sleek, and more data-driven technologies are equally widespread in the medical device sector. Moreover, the medical device sector — like most industrial sectors — is also besieged by competition.
Therefore, it is up to the design engineers to recognize the needs of their users and figure out ways to enhance the usability and effectiveness of a system or device, while simultaneously conforming to rigorous regulatory and quality standards.
One thing is for certain: the potential to determine interface pressure — be it at the time of the design process, the development process, or as an integrated feature within the device itself — can give way to new, stimulating opportunities for developments.
Being an established tool, pressure mapping technology is used for capturing real-time interface pressure insights between almost any pair of mating surfaces. Regardless of the intended end user, minimally invasive pressure mapping technology provides the perfect solution to deal with the never-ending demands for better treatment methods and medical devices.
This article demonstrates the utility of pressure mapping technology for physicians, medical device manufacturers, and medical device design engineers to realize higher standards for medical care. These instances will be endorsed by real-world pressure mapping applications.
What is Pressure Mapping?
You would find that the distribution of interface pressure — even between comparatively flat surfaces — is typically uneven within localized areas of peak pressure. Through pressure mapping technology, design engineers will be able to gain a better understanding of areas that may have an impact on both quality and design.
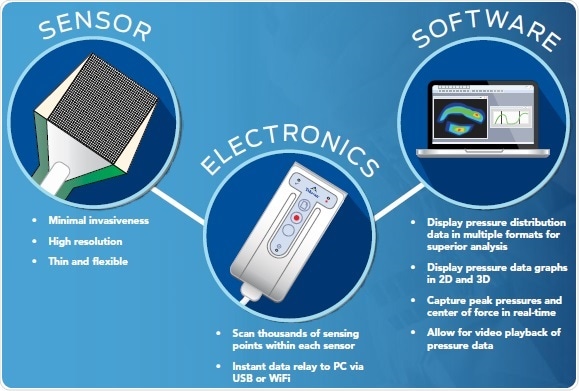
Components of a pressure mapping system
Three components — scanning electronics, sensors, and software — are required by pressure mapping systems to deliver actionable and real-time data in ways that cannot be achieved through other techniques.
- Compressive pressure loads are converted to a change in resistance by the sensor
- The real-time activity of the sensor area is displayed by the software, enabling users to visualize contact area, pressure, force, and timing data
- Analog data from the sensor are collected by the scanning electronics, which then transform these data into a digital signal
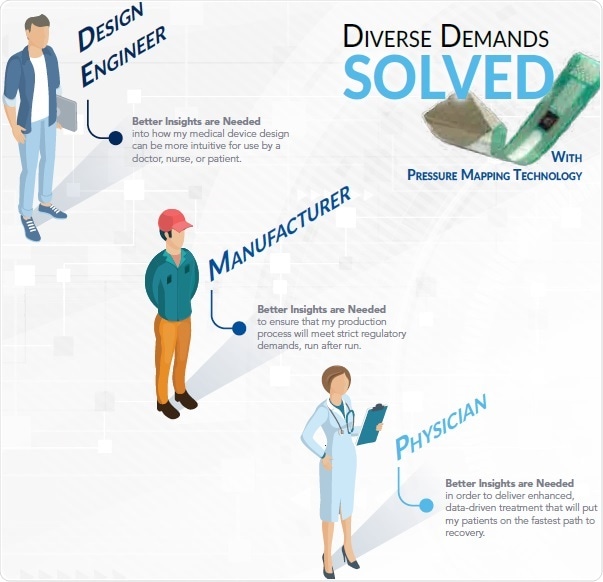
There is a Solution for Everyone
A number of challenging demands exist for medical devices that start with the design engineer and end with the doctor and their patient. In this regard, pressure mapping technology has unlimited applications to meet the end-to-end demands of each of these user groups.
About Tekscan
Amazing insights and innovative products using Tekscan pressure mapping, force measurement, and tactile sensors
Tekscan's patented tactile force measurement and pressure mapping solutions provide you with actionable information to optimize your product designs or improve clinical and research outcomes. Our sensors and systems are used in a wide range of applications either as a stand-alone solution or as an embedded technology. Simply put, we help you create better, differentiated products and services.
Located in the Innovation District of Boston, Tekscan has been in business since 1987. During that time, we've perfected our product, our team and our process to ensure we meet our customers' expectations. Today, the team is over 100 employees strong.
Tekscan: your trusted technology partner.
Quality - Tekscan, Inc. is committed to quality. Sensor design, manufacturing, and production happen at ISO 9001 compliant & 13485 registered Tekscan headquarters.. Our Technical Team is made up of electrical and mechanical engineers who consult directly with customers throughout each step of the sensor design process, from conception through production.
Innovation - Tekscan is a high technology company always pushing the limits through continuous innovation to maintain its world leading position in the area of tactile sensing. The company invests over 14% of its revenues on R&D and Innovation, similar to Google and Microsoft, and higher than the industry averages of Health & Medical (12%) and Computer & Electronics (8.8%) and much higher than the average US company (under 5%).
Company Growth - Tekscan has experienced continued growth. While we are a US company, half of our business is international. Tekscan has earned a ranking on the Inc. 5000, the business magazine’s annual listing of the fastest-growing private companies in America.
Longevity - Thanks to standing on solid financial ground, Tekscan has been able to dedicate significant resources and money to invest in itself through expanded production capabilities. Because we have no debt as a company, our customers can be confident they are making a smart and solid investment when they choose Tekscan.
We have a solution for you.
A Tekscan product is found in many different environments; in your local clinicians’ office, in an academic setting for research, a Fortune 500 company and your local retailer. Tekscan has a broad product portfolio to meet the needs of our customers. Tekscan's philosophy is to provide highly cost-effective upgrade paths to enhance and expand the utilization of our products by our customers. We have successfully integrated our technology into OEM products, and continues to develop sensors and systems that are rapidly becoming industry standards.
Sponsored Content Policy: News-Medical.net publishes articles and related content that may be derived from sources where we have existing commercial relationships, provided such content adds value to the core editorial ethos of News-Medical.Net which is to educate and inform site visitors interested in medical research, science, medical devices and treatments.