Thanks to their safety, ease of application and ability to administer a precise dose of an active pharmaceutical ingredient (API), orally disintegrating films make an appealing method of dosage. The ODF forms also enable greater bioavailability of the drug, as well as allowing circumvention of the first pass effect.
A number of viable methods exist for the production of ODFs. Hot-melt extrusion is one of the most recommended of these as a continuous process with good reproducibility which is effective without the need for added solvents.
It is possible to manufacture high quality ODFs at either a lab or production scale with the help of Thermo ScientificTM pharmaceutical extruders and down-stream equipment.
Manufacturing Methods for ODF
Two of the most common methods of production for ODFs are solvent casting and hot-melt extrusion (HME)1. For initial studies and excipient screening, solvent casting is a frequently selected option. However, while it is highly appropriate for thermolabile APIs, it does require the use of a solvent and problems may be experienced during scale-up.
An improved alternative exists in HME. Unlike solvent casting, HME is an environmentally friendly technology with no need for solvents. It offers high reproducibility, as well as demonstrating superior uniformity of content with a lower number of production stages and decreased costs2.
In addition to this, with HME, API and excipients are mixed on a molecular level, which leads to a more uniform dispersion of the API in the ODF, subsequently increasing the drug’s bioavailability.
Scale-up in HME has been well established4, and is easily carried out with Thermo Scientific extruders. With an appropriate choice of excipients, HME is the most highly recommended method for pioneering ODF formulation2.

Figure 1. Benchtop system including a Pharma 11 Twin-Screw Extruder for production of ODFs.
Hot-Melt Extrusion
HME is a well understood and established method of production which has a lengthy history in plastic and food processing and is experiencing increasing popularity in the pharmaceutical industry. Pharmaceutical formulations for HME typically consist of combinations of API, polymers and largely, plasticizers or other excipients.
HME works by melting the polymer in a twin-screw extruder. All ingredients are mixed together and kneaded. As such, intense compounding occurs. The die is positioned at the end of the twin-screw extruder and dictates the shape of the extrudate. The melt is squeezed through the hole in the die.
Additional continuous processing can be provided by down-stream equipment, including conveyors, pelletizers or take-off systems and cutters. With HME granules, it is possible to produce tablets with a modified drug release profile, in addition to transdermal, transmucosal or subcutaneous drug delivery systems3.
A sheet-die is employed to determine the width and thickness of the film for the production of ODFs. Since the majority of pharmaceutical polymers are somewhat brittle, it is vital that appropriate plasticizing excipients are chosen.
The extruded film should be flexible and stretchable. The extruded film is pulled at a fixed speed to ensure a homogenous thickness, with the help of a take-off and wind-up system.
A standard benchtop system (Figure 1) is comprised of a gravimetric twin-screw feeder which feeds the pre-blended material into a Thermo ScientificTM Pharma 11 Twin-Screw Extruder. The extruded film is continuously pulled by the sheet take-off. To finish, the film is wound up on a roll (Figure 2).
This process allows thin films to be produced at a consistent thickness of 100 μm. Adjusting the slit of the sheet die, alongside the pulling speed and throughput, allows for alteration of the thickness of the film.
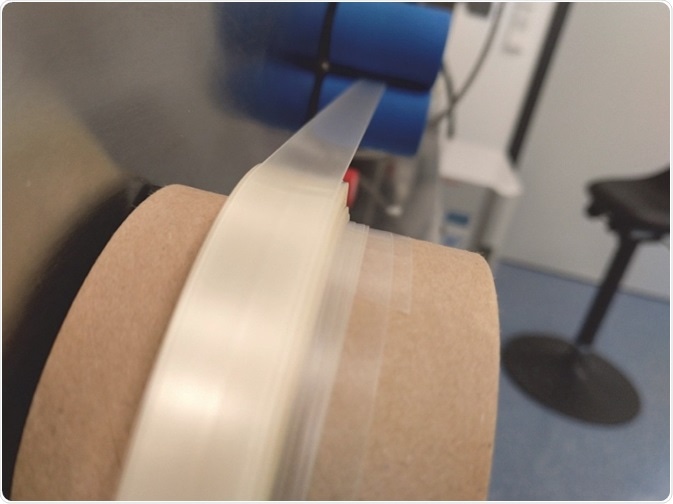
Figure 2. ODF being wound up on a roll.
References and Further Reading
- S. Karki, H. Kim, S.J. Na, D. Shin, K. Jo, J. Lee, Thin films as an emerging platform for drug delivery, Asian J. Pharm. Sci. 11 (2016) 559-574. doi:10.1016/j.ajps.2016.05.004.
- R. Jani, D. Patel, Hot melt extrusion: An industrially feasible approach for casting orodispersible film, Asian J. Pharm. Sci. 10 (2014) 292-305. doi:10.1016/j.ajps.2015.03.002.
- M.A. Repka, S.K. Battu, S.B. Upadhye, S. Thumma, M.M. Crowley, F. Zhang, C. Martin, J.W. McGinity, Pharmaceutical application of hot-melt extrusion: Part II., Drug Dev. Ind. Pharm. 33 (2007) 1043-1057. doi:10.1080/03639040701525627.
- K. Paulsen, A. Gryczke, D. Leister, Investigating process parameter mechanism for succesful scale- up of a hot-melt extrusion process, AppNote LR-71, Thermo Fisher Scientific, 2013.
About Thermo Fisher Scientific – Materials & Structural Analysis
Thermo Fisher Materials and Structural Analysis products give you outstanding capabilities in materials science research and development. Driving innovation and productivity, their portfolio of scientific instruments enable the design, characterization and lab-to-production scale of materials used throughout industry.
Sponsored Content Policy: News-Medical.net publishes articles and related content that may be derived from sources where we have existing commercial relationships, provided such content adds value to the core editorial ethos of News-Medical.Net which is to educate and inform site visitors interested in medical research, science, medical devices and treatments.