Manufacturers of catheters usually test their products by simulating real-world conditions. The simulation includes replication of a patient lying in an operating room and a surgeon inserting a catheter. ZwickRoell developed a horizontal testing machine in close cooperation with customers and surgeons. The machine has a number of special adaptations to enable the simulated operational procedure on the patient.
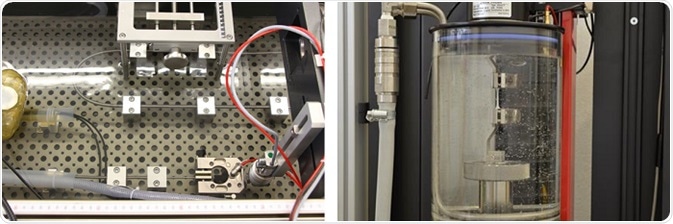
Image credit: ZwickRoell Gmbh Co. KG
Over the years, ZwickRoell has expanded its portfolio and now offers a variety of testing solutions connected to design, testing, and production challenges faced by catheter manufacturers today.
Solutions
The solutions offered by ZwickRoell vary from a horizontal Allround with or without water bath to a horizontal zwickiLine, and can determine numerous results, such as:
- Track force - the force needed to advance a catheter, interventional device or guide wire through a tortuous path is measured
- Push efficiency - the amount of force that is experienced by the distal tip of the product when a known force is being applied to the product on the proximal end is measured by the proximal and distal load cell
- Insertion force measurement - measures the force used to advance through the introducer sheath
- Guide-wire movement – provides measurement of the force needed to advance a guide wire through catheter, guide catheter or other interventional device
- Flexibility - measures ability of the tip of the catheter to track over a specified bend in a guide wire, i.e. 90 degrees
- Guide wire and catheter lubricity track measurement - comparative test which uses the track test data in order to determine if coatings have an effect on the force required to advance product through a tortuous path
About ZwickRoell, LP
With over 160 years of expertise in materials and components testing, ZwickRoell delivers intelligent materials testing solutions for the medical industry, including drug delivery testing systems for syringes and cartridges, autoinjectors, and pen injectors. Our qualification services help ensure compliance to your industry’s stringent testing requirements.
Worldwide Presence
ZwickRoell, LP is a member of the ZwickRoell Group, a key player in the global market for materials testing systems. Operating in 56 countries worldwide, the ZwickRoell Group has manufacturing facilities in Germany, the UK, Austria, and China, well as subsidiaries in France, the UK, Spain, the USA, Brazil, Turkey, Singapore, and China.
Sponsored Content Policy: News-Medical.net publishes articles and related content that may be derived from sources where we have existing commercial relationships, provided such content adds value to the core editorial ethos of News-Medical.Net which is to educate and inform site visitors interested in medical research, science, medical devices and treatments.