By storing and distributing a wide array of biospecimens and the clinical information linked to the samples, biobanks play a crucial role in biomedical research. Tracing these biospecimens and the data efficiently and accurately can be a challenge when taking into consideration the choices for processing technologies, the diversity of sample types, and the variety of storage conditions associated with the biospecimens.
Consent tracking and ethical factors introduce added complexity, needing additional steps during the process. Patient privacy and security must be considered carefully, with maximum care given to protecting the personal health information of participants who are enrolled in a study.
The process must also consider compliance with regulations relating to electronic signatures, audit trails, and chain of custody. This is because these impose additional data capture requirements during the process.
By supplying built-in workflows, better data accuracy, electronic data capture, increased process efficiency and enabling compliance with regulatory requirements, a purpose-built, commercial-off-the-shelf (COTS) Biobanking LIMS solution can help address many of these challenges.
Typical challenges encountered include:
- Tracking the chain of custody and the patient consent associated with a sample
- Managing patient privacy data securely to ensure compliance with regulatory agencies
- Efficiently store and track the biospecimens and the associated clinical data
Advantages of a purpose-built COTS LIMS are shown in the graph.
Specimen accessioning
The ability to record specimen and subject information using controlled vocabulary ensures everyone is accessioning the samples using the same language, including microscopic diagnosis, tissue types, organs, clinical diagnosis, metastasis and species.
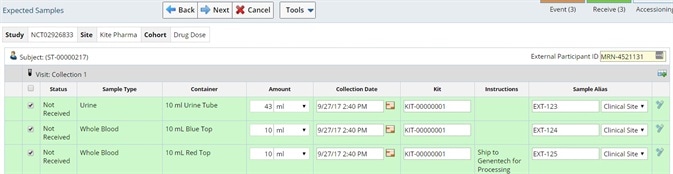
Image Credit: LabVantage Solutions
Visualization of storage inventory
A view of the lab's floor, showing each freezer and the boxes/plates that are stored in it, can help to optimize storage space.
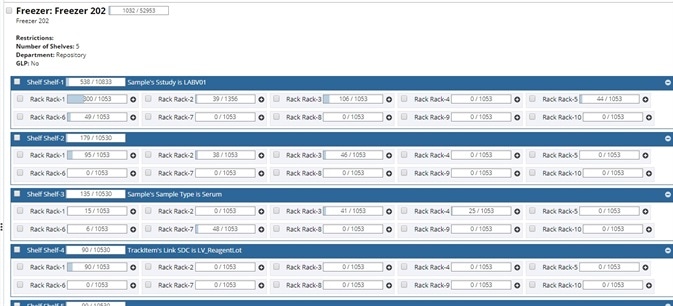
Image Credit: LabVantage Solutions
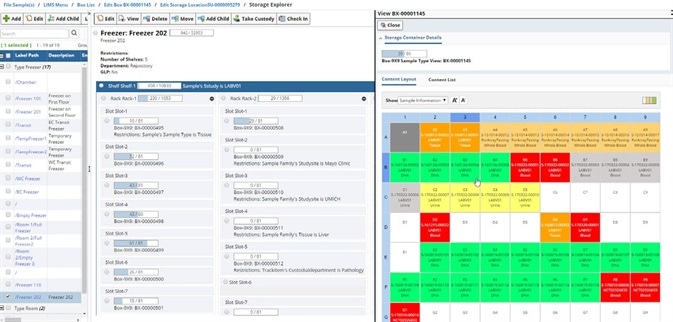
Image Credit: LabVantage Solutions
Typical biorepository process
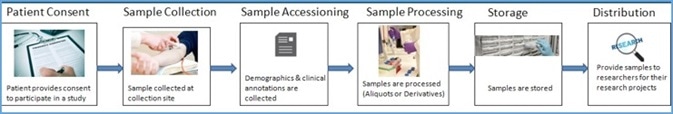
Image Credit: LabVantage Solutions
Built-in workflows
Pre-configured workflows which have been developed especially for biobanks help to improve efficiency by guiding lab managers and lab technicians through their daily tasks.
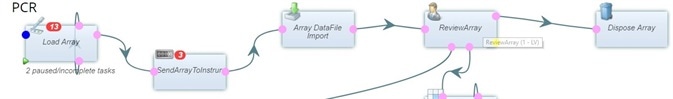
Image Credit: LabVantage Solutions
Consent management
Being able to track patient consent information ensures that biological specimens are handled in accordance with donor preferences and as per regulatory requirements.
Request management
Researchers approach biobanks when they require a specimen with certain traits for their research. A LIMS solution is able to facilitate sample sharing by supplying an interface for researchers to search specimens, submit requests and track all requestor correspondence. It can also help biobanks to manage specimen requests and process them in order of their priority.
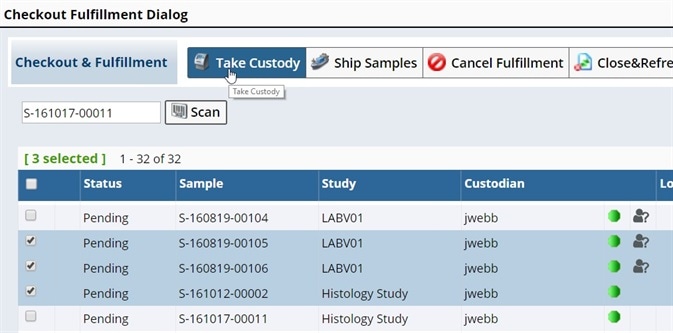
Image Credit: LabVantage Solutions
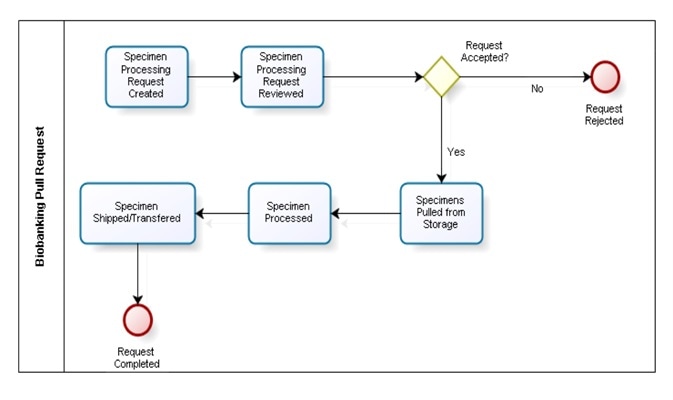
Image Credit: LabVantage Solutions
Genealogy viewer
Protocol-driven specimen collection, storage, handling, aliquoting and testing, plus the ability to track complete genealogy of derivatives, aliquots, and pooled samples fully complements biorepository activities.
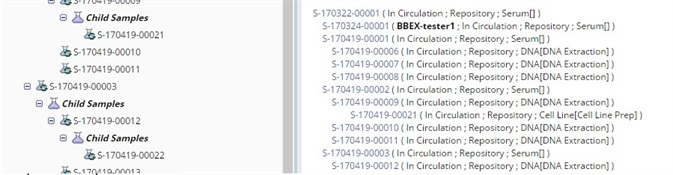
Image Credit: LabVantage Solutions
Privacy and security
By supplying the ability to capture electronic signatures along with audit trials and maintaining patient privacy while ensuring chain of custody, a LIMS enables compliance with 21 CFR Part 11 and HIPAA.
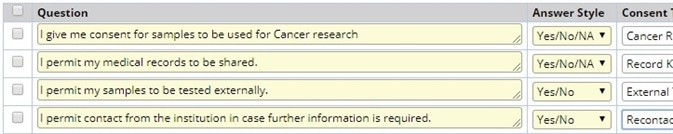
Image Credit: LabVantage Solutions
Conclusion
A LIMS which is specifically designed for biobanks, such as LabVantage 8.2 from LabVantage Solutions, resolves most of the informatics challenges inherent in biobanking. It provides a standardized solution to meet the data management needs of both small laboratories and large biobanks while improving sample traceability and enabling compliance with strict regulatory requirements.
Acknowledgments
Produced from materials originally authored by Terrence R. Smallmon, MBA from LabVantage.
About LabVantage Solutions
LabVantage Solutions, Inc. is the leading global laboratory informatics provider. Our industry-leading LIMS and ELN solution and world-class services are the result of 35+ years of experience in laboratory informatics. LabVantage offers a comprehensive portfolio of products and services that enable companies to innovate faster in the R&D cycle, improve manufactured product quality, achieve accurate recordkeeping and comply with regulatory requirements.
LabVantage is a highly configurable, web-based LIMS/ELN that powers hundreds of laboratories globally, large and small. Built on a platform that is widely recognized as the best in the industry, LabVantage can support hundreds of concurrent users as well as interface with instruments and other enterprise systems. It is the best choice for industries ranging from pharmaceuticals and consumer goods to molecular diagnostics and bio banking. LabVantage domain experts advise customers on best practices and maximize their ROIs by optimizing LIMS implementation with a rapid and successful deployment.
Sponsored Content Policy: News-Medical.net publishes articles and related content that may be derived from sources where we have existing commercial relationships, provided such content adds value to the core editorial ethos of News-Medical.Net which is to educate and inform site visitors interested in medical research, science, medical devices and treatments.