In this interview, Byron Hartman, Microscopy Product manager at Miltenyi Biotec, talks to NewsMed about automated 3D imaging performed on a larger scale than ever before.
Please can you introduce yourself, Miltenyi Biotec, and the UltraMicroscope Blaze?
I am Byron Hartman, Microscopy Product Manager for Miltenyi Biotec. Our new product is the UltraMicroscope Blaze, a light sheet fluorescence imaging system that enables automated 3D imaging on an unprecedented scale. This instrument is the next generation of light-sheet imaging technology and builds upon more than a decade of development of the Ultramicroscope series, which has been highly impactful in biomedical research and clinical applications for whole organ imaging.
UltraMicroscope Blaze™ – Understanding natures complexity
For 30 years, Miltenyi Biotec has played an important role in the development of products that empower the advancement of biomedical research and enable cell and gene therapy. LaVision BioTec was founded 20 years ago and has become a leading global specialist for advanced light-sheet and multi-photon microscopy. LaVision BioTec joined the Miltenyi organization in late 2018 and, with this fusion of expertise and resources, Miltenyi Biotec now offers a suite of innovative products that successfully address the high-end microscopy market, serving applications in neuroscience, oncology, immunology, and more.
Miltenyi Biotec's biomedical research and imaging technology support a complete workflow including imaging instrumentation such as light-sheet microscopy, multi-photon microscopy, and high-content imaging, as well as solutions including antibodies and tissue clearing reagents, sample preparation support, and more.
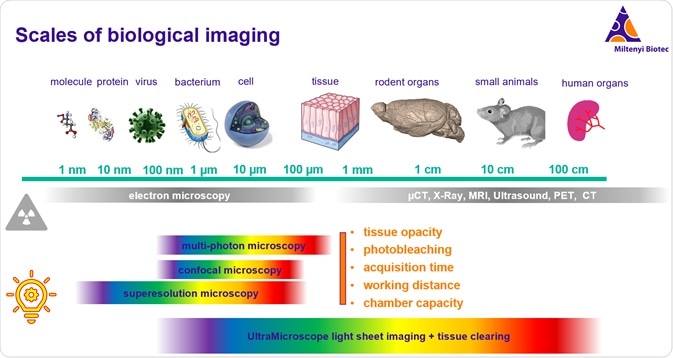
Imaging across the scales of biology is a challenge in basic sciences and clinical research. Electron microscopy allows us to image at the smallest scales. Other modes of radiation-based imaging such as CT, X-ray, MRI, and ultrasound allow us to image at the organism level.
Meanwhile, fluorescence microscopy has been incredibly potent in basic scientific research because of the ability to use the spectral properties of light to multiplex imaging and to take advantage of fluorescent proteins using transgenic model organisms.
What are the limitations of traditional fluorescence microscopy methods?
Fluorescence microscopy has been limited historically by several key factors that have prevented it from being used on a larger scale. These factors include tissue opacity, photobleaching, acquisition time, optical working distance, and physical size of the imaging stage or chamber. Traditional imaging methods lead to excessive photobleaching to the point that you cannot image the entire volume without bleaching it first. Slow acquisition time has prohibited the practical use of 3D imaging for very large specimens.
However, beginning with the UltraMicroscope, the first commercial light-sheet system launched in 2009 by LaVision BioTec, many of these barriers have been addressed and pushed back. With this technological development, we have also seen an incredible advancement in tissue clearing technologies. Tissue clearing addresses the opacity barrier, while selective illumination and fast acquisition are two key properties of light-sheet microscopy that have enabled this technology to move into the large volume fluorescence imaging space. Selective illumination with light-sheet imaging and the optical and mechanical design of the UltraMicroscope series of systems addresses photobleaching, acquisition time, working distance, and chamber capacity.
This means that we now have systems that can image all the way through this range of biological interest up to the volume of a small rodent. On the same system, one can image to below one-micrometer resolution.
What sort of applications can this technology be used for?
Tissue clearing and imaging by light-sheet microscopy have become a powerful tool to study the overall composition of functional units in entire organs as well as the impact of diseases on these structures. Many different types of samples can be imaged at a large volume with systems like the UltraMicroscope series of instruments. Whole mouse brain imaging is a very strong application area, with many papers in high profile journals published with the UltraMicroscope II, looking at the mapping of neurons and glia as well as fine-scale vasculature for example. Whole brains can be labeled with antibodies like the tyrosine hydroxylase antibody. They can also be labeled with fluorescent proteins. Three-dimensional brain data can then be registered and aligned with a standard anatomical atlas, like the Allen Brain Atlas, and cell counting and brain activity such as c-fos expression can be quantified across the entire brain and many brains can be averaged for statistics.
We also see broad use of our instruments for embryogenesis, tumor analysis, cardiovascular biology, immuno-oncology, and more. There is a very wide range of applications for cleared tissue imaging at large volumes amongst our many users. The UltraMicroscope has about 300 publications to date. Many high-profile research discoveries have been made with the UltraMicroscope series of instruments and many of the current tissue clearing technologies were first developed and tested with UltraMicroscope instruments.
I can share a video for your readers that highlights some of the types of applications and data that have been explored with the UltraMicroscope instruments.
Light sheet microscopy for 3D imaging of large optically cleared samples
Why is tissue clearing important within light microscopy?
Imaging through large biological samples with light microscopy requires tissue to be “cleared” to remove opacity and normalize the behavior of light as it travels through the sample volume.
In normal living or fixed tissue, the light passage is highly impaired due to reflection, refraction, scattering, and absorption as it encounters different materials in the tissue. Water, proteins, and lipids compose most of the material in tissue and each of these has different properties that affect light passage.
The refractive index is a unitless property that represents the speed at which light travels through a given material as compared to the speed of light in a vacuum. For example, water has a refractive index of 1.3, proteins of around 1.5, and lipids of around 1.4.
This number is an expression of how much slower light moves through a substance than in a vacuum. The higher the number, the slower light travels in that material. Clearing protocols seek to normalize the refractive index within the material while preserving proteins and tissue structure.
Since water has the lowest refractive index of the materials in the tissue, it is always replaced with either organic solvents or aqueous reagents with a higher refractive index closer to that of proteins and lipids. Most protocols also have a lipid solubilization effect, which serves to remove lipids or modify them to match the reflective index proteins more closely.
Some tissues also require a bleaching step to remove light-absorbing proteins. After clearing, the tissue is immersed in a refractive index-matched imaging solution and, now that the refractive indices across the material are normalized and the tissue is immersed in a refractive index matched solution, it becomes transparent.
Miltenyi Biotec provides support and resources for tissue clearing. We recently completed a three-part live webinar series called the Tissue Clearing Webinar series, which is now available on-demand.
Miltenyi Biotec also recently launched the MACS Clearing Kit and imaging solution reagents. These are easy to use kits for the immunostaining and clearing of a variety of tissues. They include permeabilization, immunolabeling, and clearing agents and offer a non-toxic solution, optimized for processing brain hemispheres, organoids, and other types of tissue. These kits are a good place for researchers to start when they are first exploring tissue clearing technologies.
What is your preferred tissue clearing method?
The type of tissue clearing method used depends on the application. Initially, the most powerful clearing methods were organic methods based on solvents like benzyl alcohol, benzyl benzoate, dibenzyl ether, and others. Many of those methods provide exceptional clearing, but they also tend to remove all endogenous fluorescent signal, so immunofluorescent labeling is required.
If you are trying to directly image a fluorescent protein, like tdTomato or GFP, you need to stain for it with antibodies, and that can add time. It depends on whether you want to image fluorescent proteins directly or whether you want to image antibody staining or other vital dyes. Many lipophilic dyes do not work very well with organic clearing methods, for example, so aqueous methods should be considered here.
The DISCO series of clearing methods was developed mostly by users of the UltraMicroscope. The DISCO methods use dibenzyl ether to clear the tissue after dehydration, usually in methanol or another dehydrating alcohol. Then, after the clearing in dibenzyl ether, it is imaged in dibenzyl ether. Although dibenzyl ether is inexpensive and relatively easy to get, it is also toxic. You need to have a device to capture the fumes and it will eat through a lot of materials, so you have to be very careful about what kind of materials you use to handle it. Some people are now using dibenzyl ether to clear the tissue, but then they often switch to more non-toxic reagents.
Ethyl cinnamate is a non-toxic phytocompound with a very similar refractive index to dibenzyl ether of around 1.5. Ethyl cinnamate can be used to clear tissue on its own, but you can also use dibenzyl ether to clear tissue and then switch to Ethyl cinnamate for the imaging. That has become a popular method. Tissues that are already somewhat optically clear, for example, a young mouse embryo or a newborn mouse brain before too much myelin build-up, are relatively easy to clear and so you can clear those with just Ethyl cinnamate.
The answer is that it really depends on the situation. It depends on the type of sample and the type of staining. There are a lot of options.
How does light-sheet microscopy compare to confocal microscopy?
Confocal microscopy illuminates the sample with excitation light that moves down through the objective lens and passes through the sample, illuminating the entire sample volume before then being reflected back through the objective lens for detection, resulting in whole volume illumination. Confocal relies on a pinhole and on a photomultiplier tube for detecting light through a laser scanning mechanism, which takes a very long time.
Comparatively, light-sheet microscopy overcomes this limitation of slow imaging and reduces photobleaching. In confocal, when light is passed through the entire sample, lots of light needs to be thrown away because of the pinhole and you end up illuminating the entire sample volume. This causes photobleaching quickly. You cannot image through a very large volume before it bleaches.
With light-sheet microscopy, we have selective plane illumination. The illumination is not through the imaging objective lens but through a cylindrical lens orthogonal to the imaging plane. The cylindrical lens illuminates only the selective plane that is needed for imaging.
The acquisition is also camera-based because there is no out of focus light. With confocal, you need the pinhole and scanning system to eliminate the out of focus light. With light-sheet imaging, you can acquire the images with a high-speed CMOS camera with a high pixel resolution and a large field of view. You can rapidly acquire images about a thousand times faster than confocal; this allows you to image much faster and avoid photobleaching, accomplishing imaging of a large volume in a time that is reasonable and practical for use.
Please tell us about the features of the UltraMicroscope Blaze.
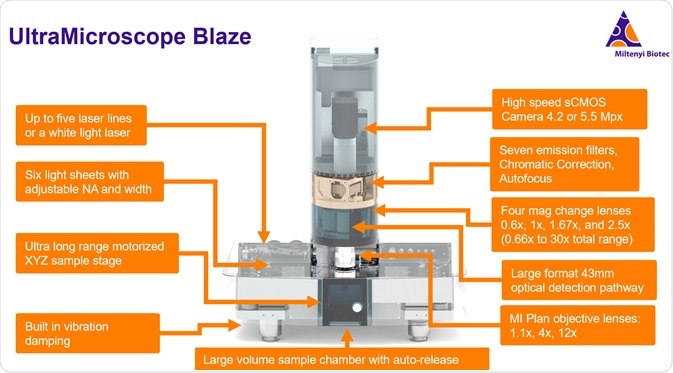
The UltraMicroscope instruments are all designed to be solvent resistant and compatible with all clearing methods. They are designed for large volume imaging with specialized optics, including an ultra-long working distance objective that dips into imaging media, and large volume imaging chambers as well as six adjustable light sheets for homogeneous illumination.
Over the past decade, the UltraMicroscope instruments have evolved into a full series of instruments. This includes the UltraMicroscope II, which is offered with either zoom optics or the SuperPlan detection system which incorporates a switchable imaging objective mount with specialized flat-field corrected optics for high performance and sensitivity. This year, we launched the UltraMicroscope Blaze which adds many automated functions including a 3-objective turret, intermediate magnification changer, massive sample capacity, batch acquisition, as well as high-level performance, and ease of use.
With the UltraMicroscope Blaze, light-sheet imaging can be viewed from a completely new perspective. Imaging is fully automated and can be performed easily on multiple samples with subcellular resolution.

The system has a tower housing the detection pathway with the camera at the top and the sample chamber at the bottom, including the objectives, intermediate magnification changers, emission filters, chromatic correction, and the opacity optical pathway. The base unit houses all the components for generating the light sheets from the left and the right side. It also houses the XYZ stage motor and the deep sample chamber.
The lasers for the light sheets can come from either LED lasers, for which you can select up to five laser lines from our custom selection, or a white light laser. The LED laser lines range between 405 nanometers and 785 nanometers. It is useful to utilize the red, far-red, and near-infrared portions of the spectrum for reduced autofluorescence and high signal to noise. The 785-millimeter laser combined with the sensitivity of the camera allows you to utilize more of the spectrum than confocal microscopy, which relies on PMTs which are not sensitive in the near-infrared range.
The UltraMicroscope series of instruments feature a sophisticated light-sheet generating system in the base whereby three-light sheets are generated on the left and three are generated on the right, and you can use either or both for imaging. You can also turn individual light sheets on and off, depending on your application.
The numerical aperture of the light sheet can be changed so that the light sheet can be made thinner or thicker. It can go as thin as about four microns for thin z-sectioning, or it can be made thicker for sectioning of larger samples or at a lower resolution. The light sheets can also be fanned out in their width using a cylindrical lens.
Light sheets can be moved horizontally to improve the resolution so that you do not need to give up the thinness of a light sheet, even for a large sample. This is done by moving the light sheet across the sample and acquiring images as you go aligned with the narrow waist, then combining all the samples computationally so that the best images are merged into one.
Large samples can be acquired at high Z-resolution using the dynamic horizontal focus feature. This allows the flexible illumination of the sample for the best results. With standard light-sheet microscopy, the sharpness of the image is reduced, but with the UltraMicroscope light sheets, because of the dynamic horizontal focus and the narrow waist, a sharper image can be achieved in Z as well as optimization of optics in X and Y. This is ideally suited for 3D imaging of large samples.
The UltraMicroscope also features an ultra-long-range motorized sample stage, allowing 12.5 millimeters traveling in X, 43.7 millimeters in Y, and 25 millimeters in Z. This, combined with the large field of view, will enable you to image large samples such as a whole mouse, or multiple individual samples, such as, for example, five or six adult mouse brains.
Using the sample stage, as well as the tiling and batch acquisition features, you can image either very large samples, or you can choose individual regions of interest, such as individual samples within the field. Each of those regions can be set with specific acquisition parameters, such as channel selection and z-stack dimensions so that you can do a batch acquisition and acquire multiple datasets. This way you can set up the system at the end of the day, and then let it run overnight in order to acquire multiple data sets. This allows you to set up an experiment with several control and experimental specimens loaded simultaneously and imaged automatically in the same session. Similarly, multiple organs from the same animal can be mounted and imaged together as a batch. Tiling and batch processing allows a great deal of user flexibility and can save a lot of time.
The UltraMicroscope also has built-in vibration damping, and this allows the system to be more versatile. You may still want to use an air table if you are doing very high-resolution imaging and you are doing a lot of tiled imaging over a long period of time, but this system does allow it to be more forgiving. You may even be able to use it directly on the bench if you have a low vibration environment.
The large volume sample chamber is another great feature of the Blaze. The chamber size is 129 by 51 by 64 millimeters, which is a volume of around 450 milliliters - enough to hold an adult mouse.
Another feature of the volume chamber is that it has a mechanism for auto-release that is very helpful when swapping out samples or repositioning a sample. It can be somewhat difficult to work with samples when they are down in the chamber, and many systems require the objective lens to be withdrawn or even physically removed in order to access the sample. The auto-release feature allows the imaging chamber to be lowered with a motorized mechanism while the sample itself stays in place on the sample holder on the stage. The sample holder stage sits within the chamber and the chamber can be dropped with the push of a button. This makes the process of manipulating and changing samples very easy and quick, allowing for more productive imaging sessions.
A key feature that empowers the UltraMicroscope Blaze to have high resolution and large volume imaging is the multi-immersion planar objective lenses. These are flat-field corrective lenses with specialized dipping caps to accommodate the entire range of imaging solution from refractive indexes of water at 1.3 out to a reflective index of nearly 1.6, for some of the organic solvent reagents. The lenses have collars for adjusting the cap for the refractive index of the medium that you are using.
The system also includes an automated objective turret. You can switch the magnification objectives between the 1.1X, 4X or 12X with the push of a button. A standard Royal Microscopy Society sized objective is 20.32 millimeters in diameter, whereas our specialized objectives are much larger to allow the more efficient light collection and long working distances. Our 1.1X and 4X objectives are 42 millimeters in diameter, while our 12X objective is 32 millimeters in diameter. This large-format is important for allowing a combination of large fields of view, long working distances, and ultimately a high numerical aperture on these lenses.
The 42-millimeter diameter lenses have a surface area that is about four times that of a standard RMS objective. The objectives here are highly specialized and unique to the UltraMicroscope series of instruments, with working distances up to 17 millimeters. There are no other comparable lenses on the market. With the Blaze, all three objectives are mounted on the automated turret, while the UMII SuperPlan configuration allows for mounting a single objective at a time.
Separate from the three different objective lens magnifications, there is also an automated magnification changing turret, which enables users to further their imaging on multiple scales. It is important to note that the intermediate magnification lenses all have high numerical aperture - they do not reduce the numerical aperture of the system. This means that you can obtain overviews with low magnification, and then you can easily increase magnification without necessarily changing objectives, and acquire another higher resolution 3D volume within the initial volume. This can also be automated. You can have the system take multiple magnification settings in a batch acquisition, saving a lot of time. This is where the automation comes in for this system. Typically, you are acquiring huge data sets, which needs time. However, because of the automation and the batch acquisition, the system can essentially be running for almost 24 hours a day. At the end of the day, you can set up another batch, then come back in the morning and start working with your data.
There are seven emission filters. You can set these up depending on your needs and the fluorophores that you are working with. This system is set up for multiplex imaging. As I mentioned earlier, you can use a white light laser or five different LED lasers and you can use up to seven emission filters to accommodate pretty much any dye or fluorescent protein or fluorochrome.
We also have automated chromatic correction. When you image between channels, this is automated in the software with a motor. There is also an autofocus feature, which allows the lenses to dip in and find the sample within the volume with the push of a button.
The detection is with a high-speed camera. We have either a 4.2- or 5.5-megapixel camera available. There is a large field of view on this camera which allows the view of a very large sample. You can obtain a sub-micron resolution, allowing you to see in cellular detail.
The data from the camera as well as the metadata from all the experiments are acquired using software developed by LaVision BioTec called ImSpector. The system is easy to operate and it allows you to run the scope and generate datasets with ease.
All of these features come together to make the UltraMicroscope Blaze a highly powerful, highly automated system. What is inside is complex, but it is in a package and built in a way that makes it very user friendly.
The automated features take this light sheet system to the next level. The main features here that differentiate this system from its predecessors are that it is specialized for high throughput, large volume, and automation. This makes it a great fit for labs or institutions looking to process a lot of samples and look at large samples on a system-level scale.
What is the largest volume that can be imaged on the UltraMicroscope Blaze?
When you combine the large volume imaging chamber and the XYZ range, you can use tiling to collect a very large field. You can image a volume of just less than 40 cubic centimeters at the lower magnification - about an adult mouse body's size, minus the tail. I think that most users will find that the size is not a limiting factor at all for what they want to achieve.
To my knowledge, the imaging volume of the UltraMicroscope Blaze is by far the largest volume capacity of any fluorescent microscope ever produced. I do not know of any fluorescent imaging systems that can image anything even close to this.
How long does this imaging take and how large is the dataset?
That really depends on what you are imagining. For reference, a whole adult mouse brain could be imaged at a relatively low resolution setting in about 20 minutes or less for one channel, depending on exactly what settings you use and what kind of interval it is. That would be a fairly thick optical section. You would not have to take a very large number of sections and you could gather brain data fairly quickly with enough resolution to do a lot of things like look at large blood vessel patterning.
If you needed to look at capillaries and smaller cells, you would need to image at a finer resolution, at a higher magnification. You could image a whole-brain at about four microns isotropic resolution in X, Y, and Z. That should take around an hour and a half to two hours per channel.
With the automated features and the batch acquisition of the Blaze, this would allow you to set up five brains overnight and have them all imaged at high resolution. Even if each one took three or four hours for multiple channels at the highest resolution, you could come back in the morning have a lot of data ready.
The size of the dataset also influences this. For a small data set, it could be several hundred megabytes. A large brain dataset could be several hundred gigabytes. It is important to be able to handle that large data. The PC that operates the UltraMicroscope and runs the Inspector software is very high performance with a lot of processing power and RAM, and a solid-state drive to make things run faster.
We have to transfer the data to a secondary workstation, which is usually used for image processing, and after that, there needs to be a storage solution. We support our customers with finding solutions for all those things - these are common concerns that we address very often. We can support new users who are getting into light-sheet microscopy and who have questions about how to handle this data.
How was the UltraMiscroscope developed?
One of the first UltraMicroscope users, and the collaboration partner in the development of the Blaze, is Professor Ali Ertürk, who is currently the director of the Helmholtz Institute for Tissue Engineering and Regenerative Medicine in Munich. He took that position in 2019 and is the youngest director of a Helmholtz Institute to date. Dr. Ertürk was a postdoc in 2009 at Genentech, and he was a user on the very first UltraMicroscope that was sold by LaVision BioTec.
During his work as a postdoc, he developed and optimized tissue clearing methods, looking at traumatic brain injuries. He made a lot of advancements early on in the development of methods for 3D microscopy in neuroscience. As he has developed his own lab, he has also worked for hand in hand with LaVision BioTec and Miltenyi to help us improve our instrumentation.
Over the last five or six years, he has been one of the users of the early prototypes for the Blaze, and he was instrumental to optimize and test the system.
How did the UltraMicroscope Blaze impact Dr. Ertürk’s research?
The new microscope helped with this research as it allowed the imaging of larger samples - samples as large as a whole mouse body, or even large human organs. Using a prototype of the UltraMicroscope Blaze microscope in combination with advanced tissue clearing was very powerful in this case, because it allowed them to look at the big picture and see all details in an unbiased way, then focus on the cells and molecular mechanisms.
It can be done at once from head to toe, imaging the whole organism. With whole human organs, this microscope helps a lot in imaging the large samples. Their lab can make human organs such as the human kidney transparent. And so, for the first time, a cellular map of the kidney can be generated. The Ertürk lab is working in this direction of mapping human organs for bioprinting applications.
This research has many different applications. The lab is currently studying neurodegeneration in the whole mouse body, in the CNS, and in the peripheral nervous system. Blood vessels and lymph vessels throughout the whole body are also being studied in different disease cases, such as diabetes. Cancer, metastasis, and drug targeting in the entire mouse body are also being studied.
The next step in this research is to be able to scan the whole mouse with higher throughput - perhaps multiple mice being imaged at the same time within a few hours. Another further step they hope to achieve is to scan the whole human brain, for which light-sheet microscopy potentially needs to be looked at from an entirely new perspective.
Interview: Dr. Ali Ertürk on the new UltraMicroscope Blaze
Please can you sum up the benefits of the UltraMicroscope Blaze?
After more than 10 years of development, we are excited to have launched the new UltraMicroscope Blaze, thanks to the work of the LaVision BioTec and collaboration with users like Ali Ertürk.
The new UltraMicroscope Blaze features all of the elements that you are looking for in a high performance, large volume fluorescent imaging system. We have six light sheets to evenly illuminate the sample, automated objective lenses, and automated intermediate magnification so that you can easily carry out life sciences imaging for a large range of scales.
The ability to image large samples lets us take science into a more systems-level approach. A very recent Cell paper from Dr. Ertürk's group (Pan et al. 2020) utilized this feature to scan whole mouse bodies looking for cancer metastasis. We are very excited about many of our other users and looking forward to seeing what people will be able to do with the new UltraMicroscope Blaze.
The UltraMicroscope Blaze is flexible and easy to operate the system with adjustable light sheet illumination. The light sheet dynamics allow you to focus across the samples and sweep the sweet spot of the waste. Systems are compatible with all clearing protocols. Many of the most popular tissue clearing protocols have been developed by labs while they were working with the UltraMicroscope Blaze.
The UltraMicroscope Blaze has a particularly large sample capacity and with the new optimized optics and the dipping lenses, you can get cellular resolution on a very large scale. We are very excited about this development for the UltraMicroscope family and look forward to enabling customers to use these tools to push the limits of scale even further in fluorescent microscopy and systems-level research.
Where can readers find more information?
For more information or if you have any questions, please contact the team.
About Miltenyi Biotec
Miltenyi Biotec is a global provider of products and services that advance biomedical research and cellular therapy. Our innovative tools support research at every level, from basic research to translational research to clinical application. This integrated portfolio enables scientists and clinicians to obtain, analyze, and utilize the cell. Our technologies cover techniques of sample preparation, cell isolation, cell sorting, flow cytometry, cell culture, molecular analysis, and preclinical imaging.
Our 30 years of expertise spans research areas including immunology, stem cell biology, neuroscience, and cancer, and clinical research areas like hematology, graft engineering, and apheresis. In our commitment to the scientific community, we also offer comprehensive scientific support, consultation, and expert training. Today, Miltenyi Biotec has 2,500 employees in 28 countries – all dedicated to helping researchers and clinicians around the world make a greater impact on science and health.
Sponsored Content Policy: News-Medical.net publishes articles and related content that may be derived from sources where we have existing commercial relationships, provided such content adds value to the core editorial ethos of News-Medical.Net which is to educate and inform site visitors interested in medical research, science, medical devices and treatments.