Situation
At the height of the COVID-19 pandemic, the UMASS Memorial DCU Field Hospital was constructed to offer care for acute COVID-19 patients who did not require intensive care. The Field Hospital has admitted over six hundred patients since its inception.
During this time, staff delivering patient care worked in areas with a significant presence of SARS-CoV-2 in the environment.
Based on infection control practices, airlock systems are developed to trap in-air pathogens and prevent them from escaping from containment areas; yet, quantitative metrics are not available without an appropriate in-air pathogen surveillance solution.
This means that verification of the proper functionality of the airlock systems is not possible.
Due to the high transmission areas, the UMASS Field Hospital wanted to achieve the following:
- Monitor the Field Hospital’s cold zones – those not containing COVID-19 patients - for the presence of SARS-CoV-2; this includes areas such as staff break rooms and gown rooms.
- Help verify that the airlock containment system is effectively keeping SARS-CoV-2 confined to the hot zones (containing COVID-19 patients).
- Oversee how effective staff safety protocols are when moving between the hot zones and the cold zones.
Solution
The Thermo Scientific™ AerosolSense™ Sampler, a new in-air pathogen surveillance solution, has been developed to supply timely and highly reliable insight into in-air pathogen presence for monitoring and improving facility safety protocols.

Image Credit: Thermo Fisher Scientific – Environmental and Process Monitoring Instruments
This solution was supplied to the UMASS Field Hospital to assist in monitoring goals while facilitating the protection of its staff and maintaining operational continuity.
Samples of air are accumulated in the AerosolSense Sampler via an omnidirectional inlet. The collection substrate is carried by a sample cartridge installed into the sampler. Samples of air are then directed toward the collection substrate via an accelerating slit impactor.
Particles are confined to the collection substrate as the air circulates around the collection area. After completion of the sampling cycle, the sample cartridge is extracted and sent to a testing laboratory.
The set-up:
- Four AerosolSense Samplers were positioned in eleven different locations across the Field Hospital for a period of four weeks.
- Samplers were positioned in the cold zone, hot zone, and warm zone areas, as shown in Figure 1. The sampler locations varied across the hot zones to compensate for the density of patients and severity of illness. Samplers were not placed inside rooms with patients.
- Air samples were collected from each location at different time points (4, 6, 8 and 12 hours).
- For results within 24 hours, the samples were sent to a Thermo Fisher Scientific laboratory partner.
Results
In the air samples of the warm or cold zones, the presence of SARS-CoV-2 was not detected, verifying the effectiveness of facility safety protocols and signaling the absence of pathogen transmission across the field hospital facility.
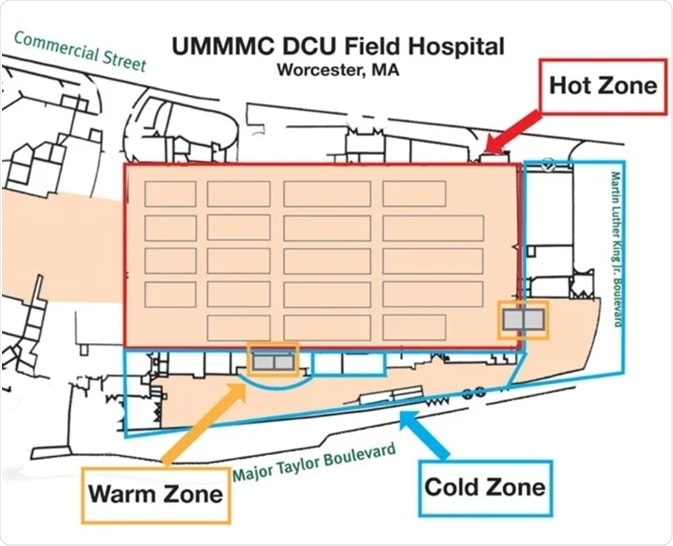
Figure 1. Field Hospital zone map. Image Credit: Thermo Fisher Scientific – Environmental and Process Monitoring Instruments
However, in a number of hot zones, in-air SARS-CoV-2 presence was detected, further establishing the necessity of infectious control measures and the criticality to follow safety protocols diligently.
A positive in-air result outside of contained areas (cold zones) could have activated a chain of actions, including staff testing, staff absence, additional cleaning measures, air handler checks, airflow checks and/or replacement of HEPA filters as well as room or wing closures.
Table 1. Field Hospital zone identification. Source: Thermo Fisher Scientific – Environmental and Process Monitoring Instruments
| UMASS Memorial DCU Field Hospital (50,000 sq. ft.) |
| Cold zone |
Warm zone |
Hot zone |
Outside air lock
- Staff break rooms
- Staff gown rooms
|
Air lock rooms
- Air lock room #1: Located next to the hot zone
- Air lock room #2: Located next to the cold zone
*Each air lock room has specific safety protocols in place.
|
Shared spaces
A highly controlled negative air pressure area with air handlers in the ceiling filter and the use of HEPA filters for expelled air. The hot zone is subdivided into five areas, based on degree of illness and density of patients (the main factors contributing to viral load).
Coughing and some respiratory treatments are the main causes of aerosolization of viral particles (even with a mask on) and contribute to the viral load in the environment. Patients are required to use a surgical mask when they walk around the Field Hospital. Inside their rooms, patients usually wear a surgical mask over an oxygen delivery device. |
| Staff only |
Staff only |
Staff and Patients |
These actions can severely disrupt daily operations; thus, the confirmation of negative results in cold and warm areas offered peace of mind. Staff were also willing to continue using the AerosolSense Sampler, particularly in staff break rooms and gown rooms, for maintaining continuity in operations.
Multiple factors contribute to local viral load across a specific period of time, leading to some degree of variability with hot zone results.
These factors include the density of patients, coughing levels, degree of illness, unequal distribution of aerosolized viral particles in the air, periods of time patients are not fitted with a surgical mask and even the shape of their face for mask fit effectiveness.
Depending on the stage of the infection, SARS-CoV-2 patients shed varying viral load levels. Other factors worth considering are the infectious control measures in the hot zone, including the negative pressure.
As the Field Hospital Director stated, “If results were uniformly positive everywhere in the hot zone, I would be concerned as that may imply negative pressure is not working.”
The AerosolSense Sampler performed as expected regardless of the airflow variations and verified that the negative pressure was indeed working.
Conclusion
AerosolSense Sampler surpassed the expectations of the UMASS Field Hospital Director.
It showed that existing infection control measures were working, helped confirm that cold zones did were free from the presence of SARS-CoV-2 (therefore reducing operational impacts) and provided the entire staff with a confidence boost in their safety protocols.

Figure 2. AerosolSense Sampler. Image Credit: Thermo Fisher Scientific – Environmental and Process Monitoring Instruments
Going forward, they anticipate that the continued use of AerosolSense Samplers in cold zones (surgical rooms, staff break rooms and gown rooms) will be a major benefit.
Hospitals have previously suffered entire unit closures after a staff member tests positive, but it is believed that the AerosolSense Sampler as a surveillance system can help prevent full unit closures.
Just the presence of the sampler has given our staff a lot of confidence because they feel we care about their safety and that we are taking the risk on getting a result that we may not like. This type of action and attention in situations like a pandemic is what people remember in the hospital system.”
Field Hospital Director
About Thermo Fisher Scientific – Environmental and Process Monitoring Instruments
 We design and manufacture industry-leading products for Gas & particulate pollutants, Flow, gas and liquid measurement, Process analytical measurements, and Industrial Hygiene.
We design and manufacture industry-leading products for Gas & particulate pollutants, Flow, gas and liquid measurement, Process analytical measurements, and Industrial Hygiene.
Technologies have proven to help customers improve efficiency, ensure process and quality control, maintain regulatory compliance, and increase worker safety.
Process mass spectrometers
Maximize product yield and increase profitability with process mass spectrometry analysis. Process gas analyzers are engineered to meet a number of challenging process applications in the petrochemical, iron, and steel, and biotechnology industries. Highly reliable and easy-to-own, Thermo Fisher Scientific process gas analysis technologies deliver faster, more complete, lab-quality online gas analysis and process analytics. Learn More
Process analytical technology/Biopharma
Process Analytical Technology (PAT) is a regulatory framework initiated by the United States’ Food and Drug Administration (FDA) that encourages pharmaceutical manufacturers to improve the process of pharmaceutical development, manufacturing, and quality control.
PAT aims to improve process efficiency by defining Critical Process Parameters (CPP) and monitoring these CPPs to stay within a defined limit, either in-line or on-line to maintain a product’s Critical Quality Attributes (CQA). Monitoring CPPs with process mass spectrometry gas analysis reduces over-processing, pinpoints contaminants, and increase product consistency. Learn More
Industrial hygiene
Industrial hygiene instruments keep your investments secure, your facilities compliant with local regulations, and your workers safe from leaking pollutants, gases, or toxic vapors. Make sure you have the most updated technology, with access to repair and calibration support to maintain reliable gas monitoring. With our environmental expertise, you can breathe easy knowing your company, workers, and neighboring communities have cleaner, safer, and healthier air quality. Learn More
Sulfur analyzers
Online sulfur analyzers replace expensive and time-consuming laboratory sampling. Get fast responses and wide measurement ranges of trace sulfur and total sulfur in flare gas, liquid, and vapor for reliable emissions monitoring. Learn More
Gas & particulate analyzers
Increasingly stringent regulatory requirements are making it more difficult to maintain regulatory compliance and optimal process performance. Air quality monitoring and reporting requirements in the U.S., China, India, Europe, and Latin America are shifting and being redefined. Together we can arrive at solutions that make sound business sense. Learn More
Flow measurement
Flow measurement and process control are critical aspects of producing, handling, and transporting hydrocarbons around the world. With our Thermo Scientific suite of flow computers and flow meters, we enable our customers to reduce cost, decrease lost material, automate, and monitor critical points in processes. From field to control room and upstream to downstream, our products provide greater control, confidence, and reliability. Learn More
Sponsored Content Policy: News-Medical.net publishes articles and related content that may be derived from sources where we have existing commercial relationships, provided such content adds value to the core editorial ethos of News-Medical.Net which is to educate and inform site visitors interested in medical research, science, medical devices and treatments.