Conventional visual techniques of detecting virus particles in solution provide a glimpse of an extremely small sampling volume, while the method of particle analysis employing dynamic light scattering generates an ensemble average of particles inside the solutions.
Virus particles incubated in the laboratory pose an issue, as they need to be grown inside cells in a media containing albumin and additional small proteins, like those inside Fetal Bovine Serum (FBS) or Minimum Essential Media (MEM) solutions.
When virus particles are emitted from the cells, the cell pieces are big and can be divided by centrifugation, however the lesser proteins of the media cannot be eliminated. Cautious choice of the distribution parameters from a dynamic light scattering experiment permits clear observation of the size distributions of virus particles in the presence of significantly smaller proteins that make up the media.
Background
Fish Viruses
There are many fish viruses that have the capacity to inflict substantial economic harm on both recreational and commercially significant fish populations. Four virus types are examined here with typical images of family virus types are outlined below.
- IHNV (Infectious Hematopoietic Necrosis Virus of the family Rhabodoviridae is a bullet-shaped virus with measurements ~70 nm wide x 170 nm long) that infects Rainbow Trout and Sockeye Salmon (Second from left).
- CTV (Cutthroat Trout Virus – a developing virus), a small round virus 25-35 nm in diameter of the Hepeviridae family; (below left)
- LMBV (Largemouth Bass Virus) is of the family Iridoviridae and is an Icosahedral shaped particle ~150 nm diameter and is found in the largemouth bass populations in the Southeast US (below right).
- ISAV (Infectious Salmon Anemia Virus) is usually found in farmed Atlantic salmon populations and has an extremely death rate. It is of the Orthomyxoviridae family, and virions are usually between 50-120 nm diameter (second from right).
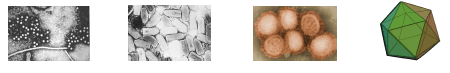
Image Credit: Brookhaven Instrument Corporation
A fast technique of assessing the existence of virus particles in solution under known incubation environments was desirable and DLS was selected as the technique to investigate.
Dynamic Light Scattering
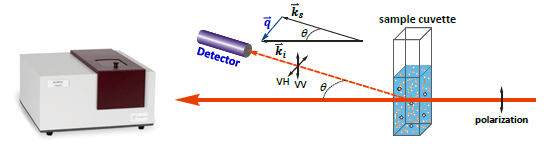
Image Credit: Brookhaven Instrument Corporation
The Autocorrelation Function

Image Credit: Brookhaven Instrument Corporation
Experimental
- Samples were kindly supplied by the USGS Western Fisheries Research Center in Seattle WA. Virus particles were grown in Chinook Salmon embryo cells in Minimum Essential Medium comprising of fetal bovine serum (FBS) and virus particles were expressed into the media. Cells and cell fragments were divided by centrifugation. Cell media with viral particles were kept refrigerated at 5 °C until measurements were taken.
- Dynamic Light Scattering (DLS) measurements were taken on a Brookhaven Instruments ZetaPALS instrument equipped with a BI-MAS particle sizing capability.
- Samples could equilibrate to 25 °C in the sample compartment for a minimum of five minutes and calculated in polystyrene cuvettes at 25 °C. DLS autocorrelation data was lowered employing the NNLS deconvolution algorithm. The decreased particle size data was assessed by both concentration and number distribution, and the outcomes exported into an Excel format and graphed in Origin 8.0.
Image Credit: Brookhaven Instrument Corporation
Distribution Curves
NNLS distributions may be weighted by surface area, number, volume, or the measured intensity. The weighting by intensity significantly highlights the largest particles due to the power relationship in diameter, where number weighting highlights the smallest particles.
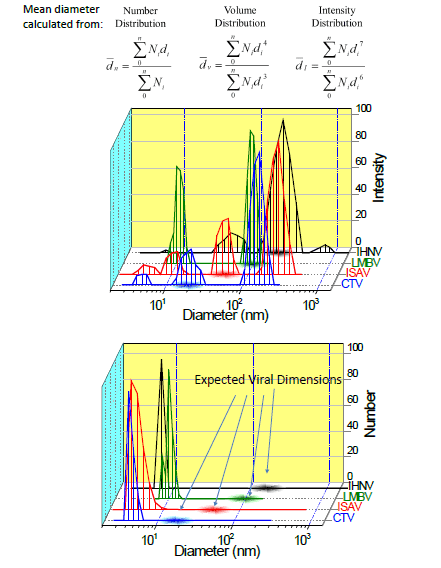
Image Credit: Brookhaven Instrument Corporation
Summary and Conclusions
The outcomes evidently display the existence of virus particles with the projected average dimensions when noted by an intensity weighted distribution, but these particles are absent in the number distribution. This indicates that the virus particles are there, but in small numbers contrasted to the proteins and additional small molecules in cell growth media.
There does seem to be some other large particles present, which may be other cell fragments or expressed molecules (RNA, proteins) that would not be likely to be eliminated in the centrifugation step because of comparable size ranges to the virus particles. Proper control samples should permit the differentiation of virus particles from free cell fragments.

This information has been sourced, reviewed and adapted from materials provided by Brookhaven Instrument Corporation.
For more information on this source, please visit Brookhaven Instrument Corporation.