Regular handwashing is vital in preventing the spread of germs and avoiding getting sick. In many situations, using soap and water to wash hands is the best way to kill microorganisms.
However, in 2002, the Centers for Disease Control and Prevention (CDC) amended the guidelines for hand hygiene to support the use of alcohol-based hand sanitizers for public and health care personnel, to limit the transmission of pathogenic microorganisms.1
Most alcohol-based hand sanitizers contain either isopropanol, n-propanol or ethanol (or a combination of two of these alcohols). The alcohol content can corrupt proteins and, therefore, demonstrates a broad spectrum of antimicrobial activity. However, the efficacy depends on the concentration of alcohol present in the solution.
Solutions that contain 60-85% ethanol and/or 60-70% isopropanol exhibit the best antimicrobial efficacy, whereas those containing less than 60% alcohol may only limit the growth of germs rather than killing them.2
As a result of the coronavirus (COVID-19) pandemic, the Food and Drug Administration (FDA) published guidelines that temporarily enabled the production of alcohol-based hand sanitizers by entities other than registered drug manufacturers.3
Yet, there are challenges and concerns to consider regarding the formulations and preparation protocols. Therefore, proper quality assurance and control measures are necessary to ensure the efficacy and safety of hand sanitizer products.
The research and development (R&D) stage is crucial for product development; it is necessary to conduct intensive research with an established R&D team as they search for the most viable product for a particular application.
By way of contrast, the production phase requires routine analysis to make sure that the product meets the requirements for final use.
The initial materials used to manufacture hand sanitizers include alcohol (active ingredient), hydrogen peroxide (disinfectant), glycerol (humectant) and sterile, distilled water - the quality control of these ingredients is critical for safety.5
Although an alcoholmeter can be used to measure alcohol concentration, this method cannot identify small contaminations, such as methanol. Instead, rapid detection can be conducted using Fourier Transform Infrared (FTIR) spectroscopy to confirm that the ingredients are up to spec.
The final product must be analyzed thoroughly to check that it contains the correct concentration of alcohol and contains no chemical and microbiological contaminants before it is sent to market.6
FTIR spectroscopy is an essential tool used across different aspects of the process, from ascertaining the chemical composition of starting materials and qualifying the purity of intermediate steps, complying with regulatory requirements and verifying the final product.
Thermo Scientific™ provides facile solutions to meet the analytical requirements from research to final production. This guide offers an overview of FTIR and the various techniques used to guarantee efficacy and safety during each step of the workflow for the production of alcohol-based hand sanitizers.
What is Fourier Transform Infrared (FTIR) spectroscopy?
FTIR spectroscopy is one of the most widely used analytical techniques employed in academia and industry to detect and quantify diverse sample components. FTIR depends on the vibration modes of the atoms of a molecule.7
When infrared radiation (IR) moves through a sample, a section of it is absorbed at frequencies that are related directly to the atom-to-atom vibrational bond energies in each molecule.
The section of the radiation that is transmitted at the detector leads to an absorption spectrum identifying the peaks where specific bond vibrations occur. Various molecules will demonstrate different peak collections, depending on their structure.
Thus, the IR spectrum presents a molecular fingerprint used to detect and determine most substances.5,6 FTIR is a non-destructive and user-friendly technique, well-regarded for its high sensitivity, selectivity and temporal resolution.
Numerous sampling techniques are also available, which opens its application to an extensive range of samples, including liquids, solutions, solids, powders, pastes, films, fibers and gases.7
The relatively low cost of FTIR instruments means that the technique can be accessed by most research centers and companies where it is generally used for compound identification, determining substances in a mixture and process monitoring.
As a result, FTIR spectroscopy is considered an invaluable technique in the chemical, pharmaceutical, petrochemical, polymer, food and catalyst industries, among others.
Research
Prior to hand sanitizers reaching the public domain, they must undergo intense research and development (R&D). Research laboratories evaluate the efficacy of various hand sanitizer formulations against target bacteria, fungi and viruses.8,9
Moreover, it must be established that the formulations are safe for use. Throughout the development phase, several tests are carried out:
- Ensure the final product meets the specifications
- Confirm the absence of microbiological contamination
- Evaluate the purity and concentration of the starting and final products
- Test the safety and efficacy of various formulations against pathogens
Thermo Scientific can provide a wide range of instrumentation that can be utilized during R&D phases. The Thermo Scientific™ Nicolet™ iS50 FTIR spectrometer offers a high level of flexibility in an all-in-one workstation for material analysis, with purpose-built accessories and integrated software (Figure 1).

Figure 1. Thermo Scientific™ Nicolet™ iS50 FTIR spectrometer. Image Credit: Thermo Fisher Scientific – Materials & Structural Analysis
The Nicolet iS50 FTIR spectrometer can be optimized to a fully automated multi-spectral range system that can obtain spectra from far-infrared to visible, using advanced attenuated total reflectance (ATR), Raman and near-infrared (NIR) modules.
It is fitted with an integrated ATR accessory and the Thermo Scientific™ OMNIC™ Software platform, which offers a simple solution for meeting the analytical requirements.
QCheck spectral correlation tool, a function of the Thermo Scientific™ OMNIC™ Paradigm software, is perfect for validating incoming materials, in-process materials or finished products.
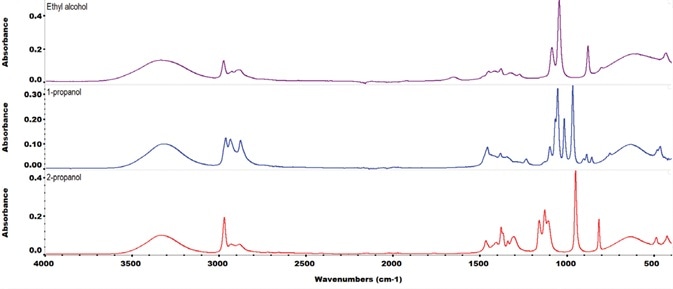
Figure 2. ATR FTIR spectra of three alcohols commonly used in alcohol-based hand sanitizers: ethanol, 2-propanol and 1-propanol. Each spectrum is 16 scans co-added at a spectral resolution of 4 cm-1. Image Credit: Thermo Fisher Scientific – Materials & Structural Analysis
Figure 2 exhibits the Thermo Scientific™ Nicolet™ iS50 FTIR spectrometer spectrum of denatured, industrial-grade ethanol when contrasted against the spectrum of the non-denatured, reagent-grade ethanol.
The denatured ethanol spectrum exhibits a peak at 950 cm-1 that relates to isopropanol. The QCheck tool demonstrates that the level of isopropanol impurity is greater than the predefined threshold.
This result was obtained without creating libraries or complex spectral manipulations, saving both time and resources.
Without the QCheck tool, the end-user would have to gather reference spectra of every possible contaminant, create their own library and contrast the sample spectrum with all the reference spectra. These procedures are time-consuming and not very efficient.
Another function of the OMNIC software is the QC Compare Search which is useful in the R&D phases of hand sanitizer. This tool is a spectral classification method that establishes the best possible match of a sample from a reference spectrum.
It also signals how similar the unknown sample spectrum is to the selected standard (that is the match value). The WHO advises that only United States Pharmacopeia (USP) or Food Chemical Codex (food grade) glycerol should be used in formulations.5
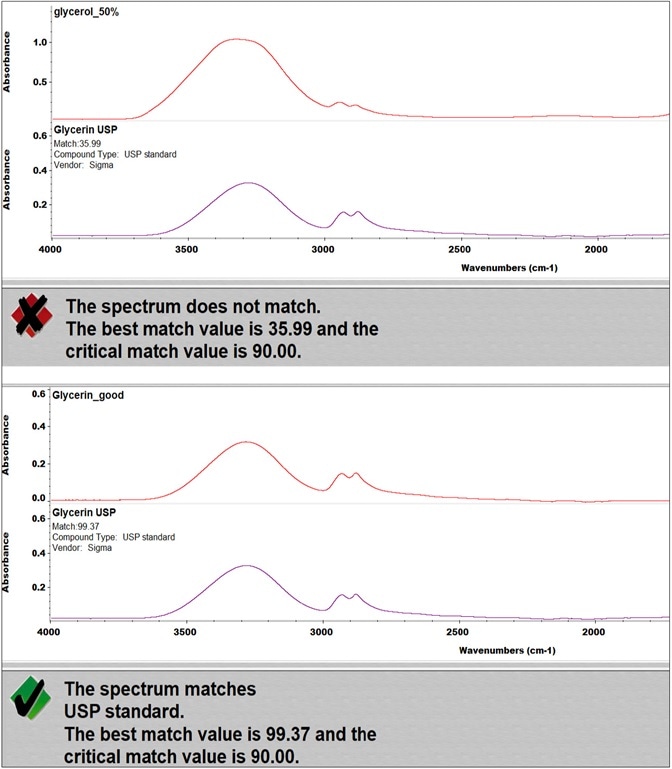
Figure 3. QC Compare results of two types of glycerol. Image Credit: Thermo Fisher Scientific – Materials & Structural Analysis
Figure 3 represents the results of the incoming inspection of two kinds of glycerol using the QC Compare function of OMNIC. The QC Compare algorithm enables the setting of a critical match value, which is the threshold that the sample similarity should overcome.
In this case, with a critical match value set at 90, one glycerol met the specified requirements and, therefore, was used for hand sanitizer production, while it was determined the other sample could not be used as it failed the test.
On top of starting material analysis, FTIR can be utilized for the quantitative determination of the alcohol fraction in hand sanitizers.
The Thermo Scientific™ TQ Analyst™ Software is well-suited for this task since it has a natural approach to sample identification, verification and qualitative and quantitative analysis.
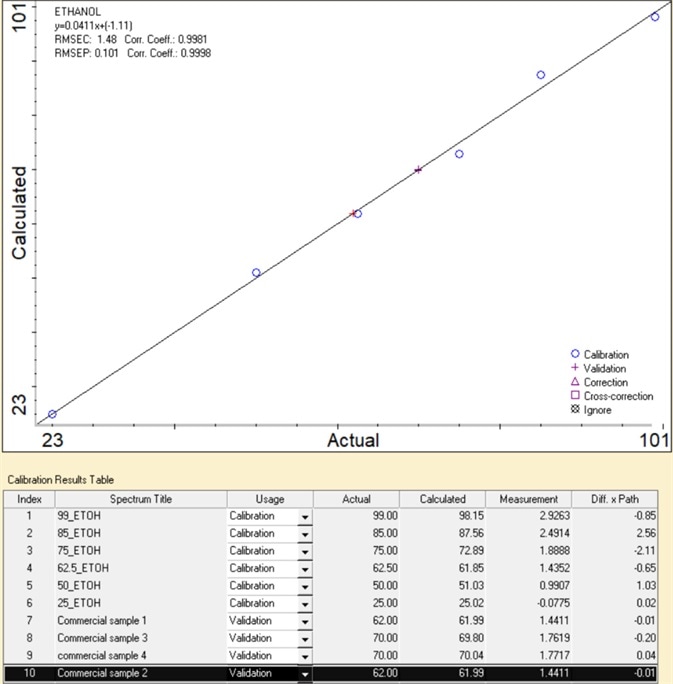
Figure 4. Calibration result of ethanol% measurements. Image Credit: Thermo Fisher Scientific – Materials & Structural Analysis
For instance, a series of FTIR measurements of ethanol/water standards with ethanol ranging from 25- 99% (v/v) were performed to establish the concentration of commercial samples (results displayed in Figure 4).
The area of the characteristic peak of ethanol at 878 cm-1 was used to create a calibration curve using Lambert-Beer’s Law in Thermo Scientific™ TQ Analyst™ Pro Edition software.
The calibration curve offers excellent linearity with a correlation coefficient greater than 0.99. This linearity is crucial because it establishes the range necessary for the acquisition of accurate and precise results.
The calibration curve can be utilized to define the ethanol percentage in samples with high accuracy. Here, four commercial ethanol-based hand sanitizers were tested.
The ethanol concentration predicted was remarkably similar to the label (with an error of less than 0.3%), which shows the quantitative capability of the TQ Analyst Pro Edition software.
Production QA/QC
Quality assurance (QA) and quality control (QC) are key components for ensuring that customers receive products compliant with specific standards and regulations.
Throughout QA/QC analysis for hand sanitizer production, it is critical that quick and easy-to-use methods exist for determining sample components so that key decisions can be made swiftly.

Figure 5. Nicolet Summit PRO FTIR Spectrometer with Everest ATR accessory and on-board touchscreen monitor. Image Credit: Thermo Fisher Scientific – Materials & Structural Analysis
The Nicolet iS50 FTIR spectrometer is too advanced for routine QA/QC analysis. Therefore, the Thermo Scientific™ Nicolet™ Summit PRO FTIR spectrometer is recommended for QA/QC laboratories as it delivers quick pass/fail results by limiting the measurements steps.
The neatly designed Nicolet Summit FTIR spectrometer has a high-performance optical engine, a built-in computer and an LED light bar for visual recognition.
Furthermore, the Nicolet Summit PRO FTIR spectrometer can be used in conjunction with the Thermo Scientific™ Everest™ Diamond ATR accessory for standard QA/QC in the production of alcohol-based hand sanitizers.
This accessory incorporates a monolithic diamond ATR crystal in combination with four single-bounce crystal plates. The Everest accessory utilizes a smart chip combined with OMNICParadigm software to recognize the accessory automatically and enhance the measurements.
Since ATR offers an accurate and non-destructive sampling method without the need for sample preparation, it is perfect for the rapid and precise analysis of incoming materials or finished products.
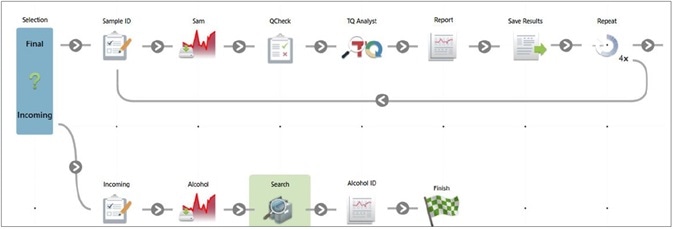
Figure 6. Workflow to verify the final product and to confirm incoming alcohol identification. Image Credit: Thermo Fisher Scientific – Materials & Structural Analysis
Confirmation of alcohol-specific materials
This section details the three applications that were developed on the Nicolet iS50 FTIR spectrometer and quickly deployed to the Nicolet Summit FTIR spectrometer.
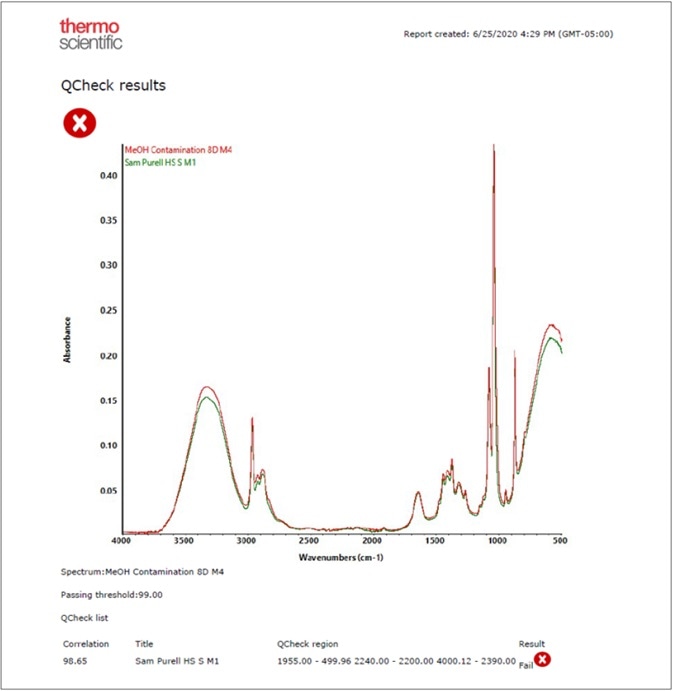
Figure 7. Results of applying QCheck to a methanol adulterated (red trace) hand sanitizer sample. Image Credit: Thermo Fisher Scientific – Materials & Structural Analysis
An OMNIC Paradigm software workflow can be utilized to determine any incoming alcohol by combing search, QCheck and TQ Analyst applications (Figure 7).
A workflow available in the OMNIC Paradigm software enables the operator to decide on the category of the incoming sample and pick from two available workflows for analysis.
Incoming Material Identification can be used to confirm that the specifications of the incoming material match the packaging. Whereas Final Product Confirmation confirms that the product has the same spectral fingerprint as an approved reference and guarantees that considerable levels of methanol are not present.
Moreover, several samples can be analyzed using the loop function present in the workflow. The QA/QC analyst must determine that the incoming material is correctly labeled – QC Compare can manage this by contrasting the sample spectrum against a reference spectrum, such as ethanol.
The Nicolet Summit can measure samples and compare them to various spectra from the Material Identification Library (i.e., ethanol, methanol, 1-propanol and 2-propanol) using the OMNIC Paradigm Software workflow.
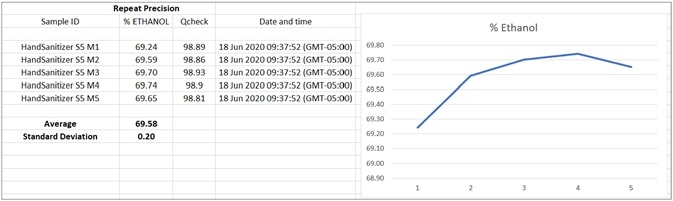
Figure 8. Chart of TQ Analyst Simple Beer’s Law method results appended to readable CSV file by OMNIC Paradigm workflow. Software like Excel or Origin may be used for statistical analysis and to build a custom control graph. Image Credit: Thermo Fisher Scientific – Materials & Structural Analysis
The Final Product Confirmation category by QCheck spectral correlation is the ideal way to confirm the quality of finished products, as they can be analyzed for contamination. Figure 8 shows a report from an experiment of a commercial hand sanitizer contaminated with methanol.
Since methanol limits the spectral correlation below the permissible threshold of 99, this adulterated sample failed to meet the QCheck finished product verification.
In addition to sample inspection, FTIR can be utilized to quantitatively identify the alcohol percentage in final products.
In one such experiment, a sequence of ethanol/water solutions with ethanol varying from 25–99% (v/v) were measured by FTIR and used to create a calibration curve using Lambert-Beer’s Law in TQ Analyst Software.
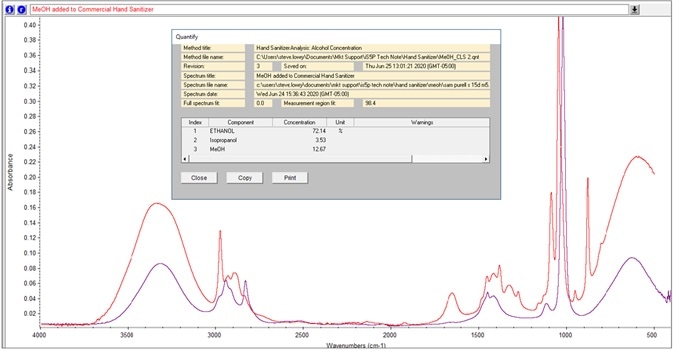
Figure 9. TQ Analyst method calculates weight percentage of methanol and isopropanol in hand sanitizer sample. Image Credit: Thermo Fisher Scientific – Materials & Structural Analysis
The calibration curve exhibits exceptional linearity with a correlation coefficient greater than 0.998. TQ Analyst software was used for establishing the precision of the measurements of five repeat ethanol-based hand sanitizer samples on the Nicolet Summit instrument (Figure 9).
A concentration of 69.58% ethanol was detected, with a standard deviation of 0.20. Another crucial step during QA/QC production is identifying and quantifying isopropanol and possible contaminants such as methanol, as it results in toxicity when absorbed through the skin or ingested.10
The isopropanol concentration must be correctly balanced; although high concentrations are not required to corrupt proteins, concentrations less than 60% will not kill germs.2
Recently, the quantification of methanol and isopropanol weight percentage in the sample was included in the TQ Analyst software. For instance, Figure 9 exhibits the analysis of a denatured hand sanitizer sample.
The term, denaturated, hints at an ethanol solution that contains additives to modify its odor and/or taste to dissuade consumption. The use of additives is severely advised against, while methanol is prohibited.5
ATR FTIR measurements take less than 30 seconds with no need for sample preparation. Swift quality control of materials at various production steps can be completed with QCheck and QC Compare, boosting consumer and production confidence in the quality of the final product.
Accurate and quantitative determination of the alcohol content in final formulations can be conducted by TQ Analyst software.
Thus, a single run of the OMNIC Paradigm workflow on the Nicolet Summit FTIR spectrometer (fitted with an Everest Diamond ATR accessory) is perfect for QA/QC of alcohol-based hand sanitizers.
Creating workflows in OMNIC
Paradigm software workflows facilitate the optimization and automation of repeatable analysis tasks, limiting errors and boosting overall efficiency.
Using the OMNIC Paradigm software, an application package containing all the workflows necessary for performing any specific task can be created.
Prior to any sample analysis, it is advised that an operator completes a performance check, crystal clean and background:
- Performance Check measures essential performance parameters of the FTIR spectrometer, such as instrument noise and spectral quality.
- Crystal Clean cleans the ATR crystal prior to analysis to limit cross-contamination.
- Background measures the signal of the instrument with no sample present. This is crucial when checking that the equipment is in good working order, and to deduct ambient noise and signals that contribute to the measurement.
The next set of workflows can be used to measure the hand sanitizer production process:
- Incoming QC confirms that the specifications of the incoming material match the alcohol content on the packaging
- Product Check confirms that the production lot has the same spectral fingerprint as the reference sample approved.
- Percent Ethanol identifies the percent of ethanol in the final product to confirm that it meets regulatory requirements.
The OMNIC Paradigm software presents a simple method when creating and editing workflows. User-defined decisions, loops, images and instructions can be merged to produce customizable workflow packages for use in specific analysis types.
The software allows end-users to access particular workflows inside a package, resulting in excellent process control and repeatable results. All these characteristics make OMNIC Paradigm software a powerful tool for QA/QC laboratories.
References
- Boyce JM, Pittet D. Guideline for Hand Hygiene in Health-Care Settings. Morb Mortal Wkly Rep. 2002;51:1-45.
- Kampf G, Kramer A. Epidemiologic background of hand hygiene and evaluation of the most important agents for scrubs and rubs. Clin Microbiol Rev. 2004;17(4):863-893. doi:10.1128/ CMR.17.4.863-893.2004
- Policy for Temporary Compounding of Certain Alcohol-Based Hand Sanitizer Products During the Public Health Emergency, Immediately in Effect Guidance for Industry, https://www.fda.gov/.
- World Health Organization. Guide to Local Production: WHOrecommended Handrub Formulations. https://www.who.int/.
- Compounding Alcohol-Based Hand Sanitizer During COVID-19 Pandemic. https://www.usp.org/.
- Stuart BH. Infrared Spectroscopy : Fundamentals and Applications. Hoboken, NJ: J. Wiley; 2004.
- Ionidis G, Hübscher J, Jack T, et al. Development and virucidal activity of a novel alcohol-based hand disinfectant supplemented with urea and citric acid. BMC Infect Dis. 2016;16(1):77. doi:10.1186/s12879-016-1410-9
- Fendler E, Groziak P. Efficacy of Alcohol-Based Hand Sanitizers Against Fungi and Viruses. Infect Control Hosp Epidemiol. 2002;23(2):61-62. doi:DOI: 10.1086/503455
- FDA Updates on Hand Sanitizers with Methanol. https://www.fda.gov/.
About Thermo Fisher Scientific – Materials & Structural Analysis
 Thermo Fisher Materials and Structural Analysis products give you outstanding capabilities in materials science research and development. Driving innovation and productivity, their portfolio of scientific instruments enable the design, characterization and lab-to-production scale of materials used throughout industry.
Thermo Fisher Materials and Structural Analysis products give you outstanding capabilities in materials science research and development. Driving innovation and productivity, their portfolio of scientific instruments enable the design, characterization and lab-to-production scale of materials used throughout industry.
Sponsored Content Policy: News-Medical.net publishes articles and related content that may be derived from sources where we have existing commercial relationships, provided such content adds value to the core editorial ethos of News-Medical.Net which is to educate and inform site visitors interested in medical research, science, medical devices and treatments.