Sponsored Content by MaxCyte, Inc.Reviewed by Maria OsipovaApr 13 2023
NK cells have a crucial role in cancer surveillance and offer a potential avenue for adoptive cell transfer as either mono- or combination immunotherapy.
Previous investigations into transferring unmodified NK cells have demonstrated their clinical safety and limited anti-tumor effectiveness.1,2
NK cells' anti-tumor activity is mediated by different mechanisms, including the antibody-dependent cellular cytotoxicity (ADCC) activated by Fc receptor (CD16) binding to antibodies.
Patients with a natural CD16 polymorphism (CD16-158V) that exhibits higher affinity for IgG1 and IgG3 have shown improved clinical outcomes following therapeutic antibody treatment against tumors.3-7
Since the CD16-158V polymorphism is present in only 10% of the population, modifying NK cells to express CD16-158V before the adoptive transfer could enhance the clinical efficacy of NK cell therapies and anti-tumor IgG1 antibodies.8
Studies performed in vitro have shown that engineered NK cell lines9 which overexpress CD16-158V have superior cytotoxicity against antibody-coated cancer cell lines, endorsing this approach.
Although primary NK cells have historically been challenging to engineer,10 non-viral engineering using MaxCyte mRNA electroporation offers a solution, with high efficiency, low toxicity, and clinical scalability, allowing for the rapid development of new adoptive cell therapy approaches.11-13
Aim
Overexpress high-affinity CD16 (CD16-158V) on NK cells as a viable combination treatment to increase anti-tumor monoclonal antibody efficacy.
Use mRNA electroporation to generate NK cells that express CD16-158V with minimal impact on cell survival and phenotype. Evaluate the effects of CD16-158V expression on rituximab-mediated cytotoxicity.
- NK cells were extracted from PBMCs from healthy donors and expanded ex vivo for 11-15 days
- Cells were resuspended in MaxCyte electroporation buffer with mRNA encoding CD16-158V
- Cells were electroporated on the MaxCyte GT using the recommended protocol.
- After electroporation, cells were resuspended in NK cell medium and transferred to culture flasks.
Complete ex vivo NK cell expansion methods and in vitro assays are detailed in Front. Immunol., 7, 105, 2016.
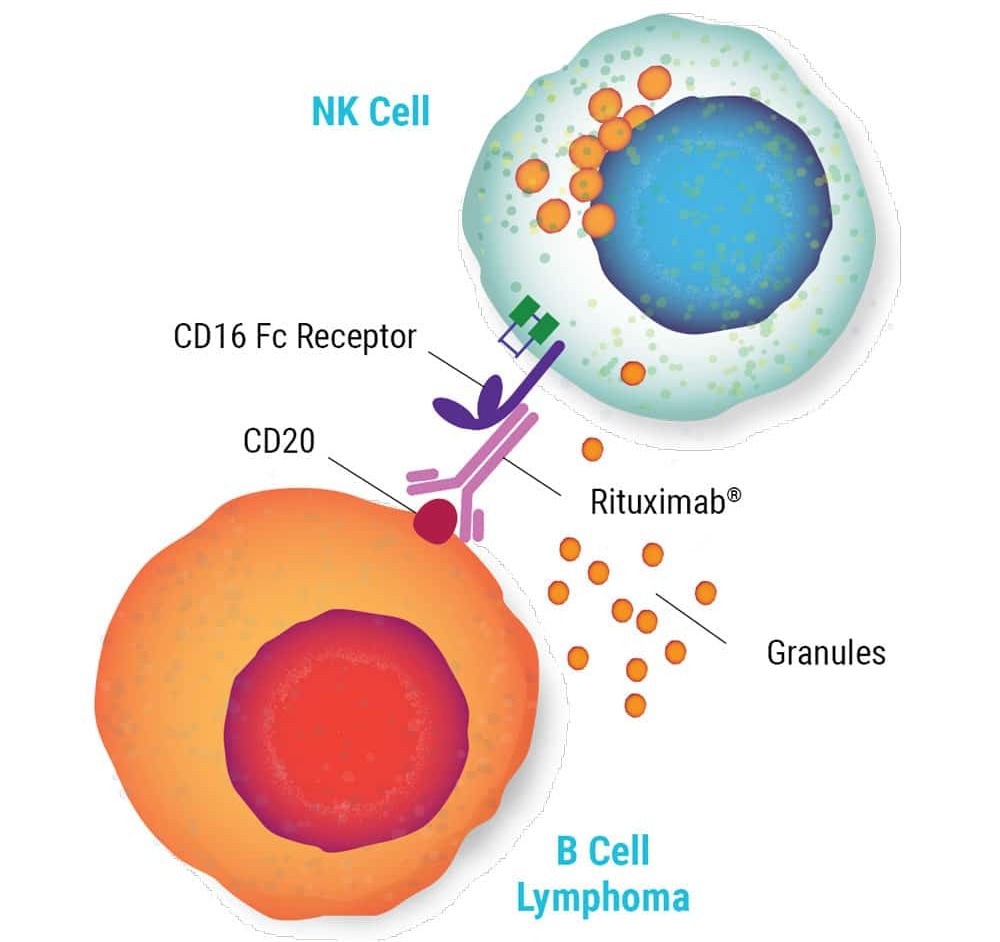
Image Credit: MaxCyte Inc. adapted from Carlsten et al. 2016 under Creative Commons License Attribution 4.0 International (CC BY 4.0)
Results
Highly efficient CD16-158V engineering of human NK cells
Ex vivo expanded NK cells electroporated with mRNA encoding CD16-158V, showed a significant rise in CD16 surface expression (Figure 1A).
The concentration of CD16-158V mRNA used during electroporation correlated with levels of CD16 expression (data not shown).
CD16 expression peaked 24 hours post-electroporation and remained higher than non-electroporated NK cells for up to 72 hours post-electroporation (Figure 1B).
Although the expression of CD16-158V was transient, this may not negate the therapeutic potential of these engineered cells because adoptively transferred NK cells have a finite persistence.
Importantly, measurement of a number of surface markers demonstrated that there were no major changes in NK cell phenotype following electroporation with CD16-158V mRNA.13
Significantly increased CD16 expression
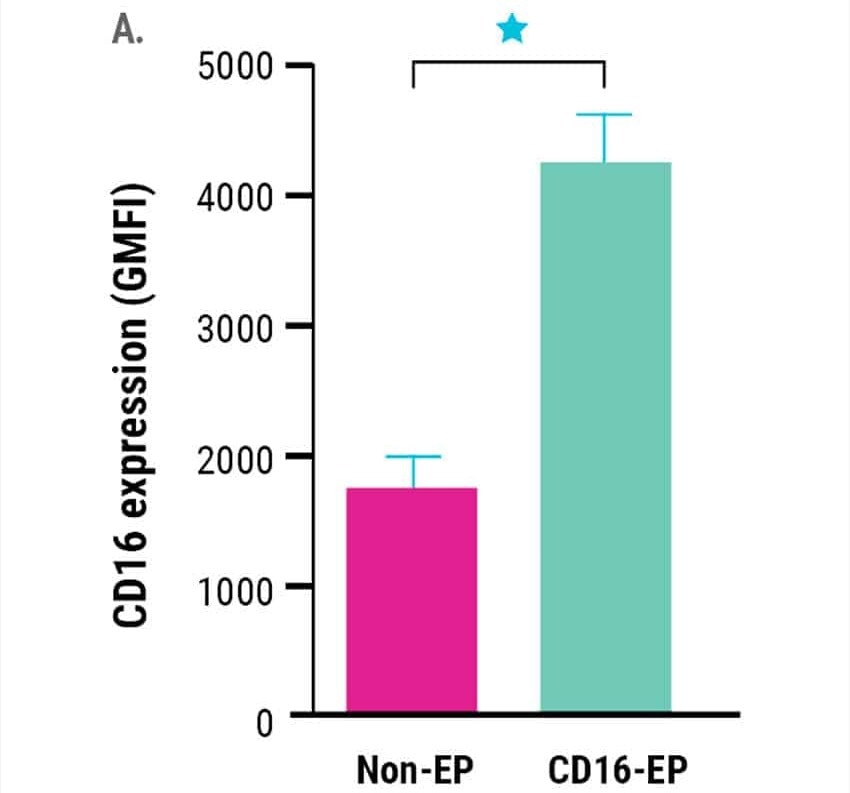
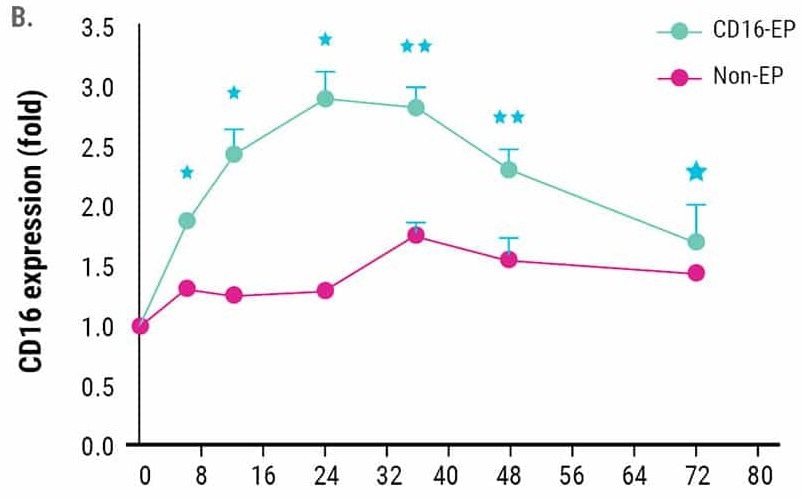
Figure 1. Ex vivo expanded NK cells were electroporated with mRNA (4 µg/106 NK cells) encoding the high-affinity, CD16-158V Fc receptor. NK cell CD16 expression was measured via flow cytometry for 72 hours post-electroporation. Non-electroporated, ex vivo expanded NK cells were used as a control to assess endogenous CD16 expression. A) CD16 expression levels 24 hours post electroporation (n=7). B) Kinetics of CD16 expression (n=3). *p<0.05, **p<0.01. Image Credit: MaxCyte Inc. adapted from Carlsten et al. 2016 under Creative Commons License Attribution 4.0 International (CC BY 4.0)
Enhanced rituximab-mediated anti-tumor cytotoxicity upon NK cell CD16-158V expression
in vitro cytotoxicity of CD16-engineered or non-engineered NK cells against rituximab-coated CD20+ B cell lymphoma cells, was measured by specific lysis of B cell lymphoma cells (51Cr release assay, Figure 2A) and NK cell degranulation (CD107a expression, Figure 2B) 24 hours post electroporation.
The engineered NK cells demonstrated an enhanced ability to mediate ADCC. This was sustained for up to three days post-electroporation, in line with the kinetics of increased CD16 expression and the concentration of CD16-158V mRNA used for electroporation.13
Significantly, mRNA electroporation did not adversely affect the non-ADCC-mediated cytotoxic function of the NK cells (data not shown).13
Augmented rituximab cytotoxicity
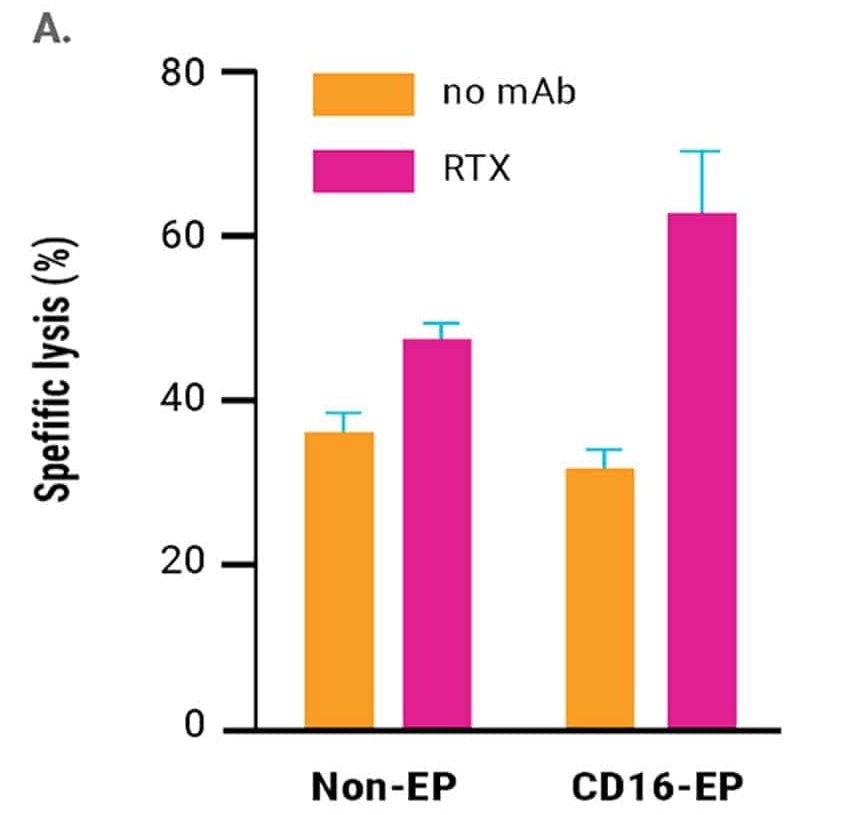
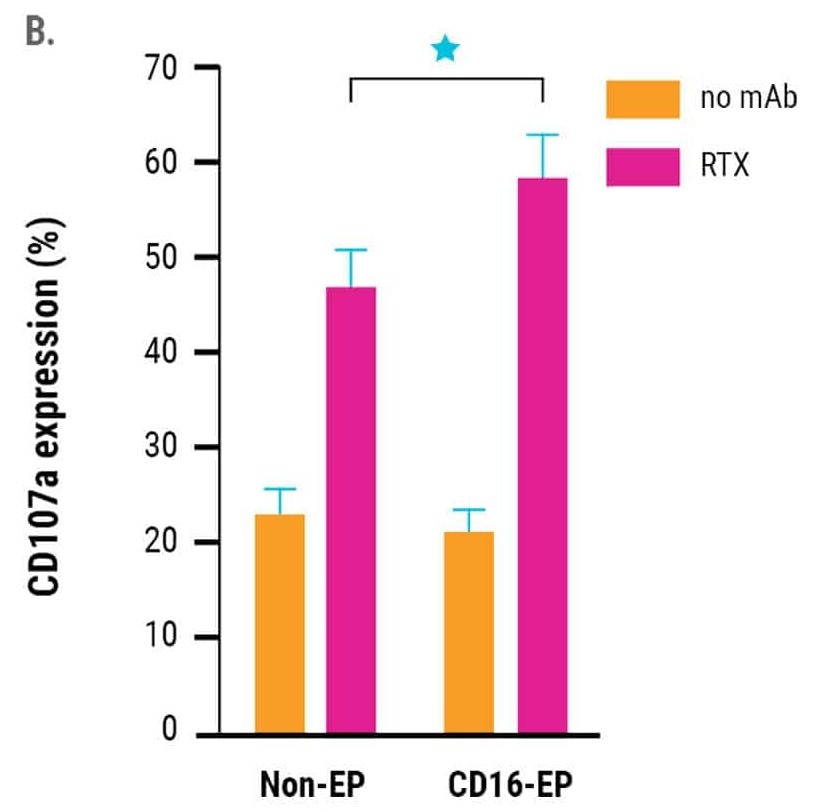
Figure 2. A) Rituximab-mediated lysis of B cell lymphoma cells was measured in a 51Cr-release assay 24 hours post-electroporation using a 0.5:1 ratio of electroporated or non-electroporated NK cells to EBV-LCL target cells. B) CD16-158V electroporated or non-electroporated expanded NK cells were cocultured with EBV-transformed B cell lymphoma cells (CD20+ 721.221 EBV-LCL cells) in the presence or absence of rituximab for 24 hours post electroporation. NK cell degranulation was assessed via CD107a expression within the CD56+ cell population. *p<0.05. Image Credit: MaxCyte Inc. adapted from Carlsten et al. 2016 under Creative Commons License Attribution 4.0 International (CC BY 4.0)
Conclusion
MaxCyte's ExPERT platform enables efficient and safe engineering of NK cells, offering a promising approach for improving the efficacy and clinical success of adoptive NK cell therapies.
The platform's unique combination of high-efficiency transfection, low toxicity, and clinical scalability enables multiplexed engineering of NK cell function, including tumor migration, cytolytic activity, and persistence.
The data presented in this article and in the primary publication demonstrate mRNA electroporation via MaxCyte's platform for rapid engineering of ex vivo-expanded NK cells, without altering their viability, phenotype, or baseline cytolytic activity.
In these preclinical studies, NK cells expressing a high-affinity CD16 Fc receptor augmented the antitumor activity of rituximab, a currently approved biotherapeutic antibody.
This cGMP-compliant, clinical-scale method of genetic modification presents an opportunity to develop commercial cellular immunotherapies and combination immunotherapies against a range of cancers.
References and further reading
- Successful adoptive transfer of in vivo expansion of human haploidentical NK cells in patients with cancer. (2005) Blood, 105:3051-3057.
- Effectiveness of donor natural killer cell alloreactivity in mismatched hematopoietic transplants. (2002) Science, 295:2097-2100.
- Therapeutic activity of humanized anti-CD20 monoclonal antibody and polymorphism in IgG Fc receptor FcgammaRIIIa gene. (2002) Blood, 99:754-758.
- Two immunoglobulin G fragment C receptor polymorphisms independently predict response to rituximab in patients with follicular lymphoma. (2003) J. Clin. Oncol., 21:3940-3947.
- Polymorphisms in FcgammaRIIIa (CD16) receptor expression are associate with clinical response to rituximab in Waldenstrom’s macroglobulinemia. (2005) J. Clin. Oncol., 23:474-481.
- Correlation of FCGR3A and EGFR germline polymorphisms with the efficacy of cetuximab in KRAS wild-type metastatic colorectal cancer. (2010) Eur. J. Cancer, 46:1829-1834.
- Rituximab infusion induces NK activation in lymphoma patients with the high- affinity CD16 polymorphism. (2011) Blood, 118:3347-3349.
- A novel polymorphism of FcgammaRIIIa (CD16) alters receptor function and predisposes to autoimmune disease. (1997) J. Clin. Invest., 100:1059-1070.
- Blocking NK cell inhibitory self-recognition promotes antibody-dependent cellular cytotoxicity in a model of anti-lymphoma therapy. (2008) J. Immunol., 180:6392-6401.
- Genetic manipulation of NK cells for cancer immunotherapy: techniques and clinical implications. (2015) Front. Immunol., 6:266.
- Expression of chimeric antigen receptors in natural killer cells with a regulatory-compliant non-viral method. (2009) Cancer Gene Ther 17(3):147-54.
- A clinically adaptable method to enhance the cytotoxicity of natural killer cells against B-cell malignancies. (2012) Cytotherapy, 14(7): 830-840.
- Efficient mRNA-based genetic engineering of human NK cells with high- affinity CD16 and CCR7 augments rituximab-induced ADCC against lymphoma and targets NK cell migration toward the lymph node-associated chemokine CCL19. (2016) Front. Immunol., 7:105.
About MaxCyte, Inc.
MaxCyte is a leading commercial cell-engineering company focused on providing enabling platform technologies to advance innovative cell-based research as well as next-generation cell therapeutic discovery, development and commercialization. Over the past 20 years, we have developed and commercialized our proprietary Flow Electroporation® platform, which facilitates complex engineering of a wide variety of cells.
Our ExPERT™ platform, which is based on our Flow Electroporation technology, has been designed to support the rapidly expanding cell therapy market and can be utilized across the continuum of the high-growth cell therapy sector, from discovery and development through commercialization of next-generation, cell-based medicines. The ExPERT family of products includes: four instruments, the ATx™, STx™, GTx™, and VLx™; a portfolio of proprietary related processing assemblies or disposables; and software protocols, all supported by a robust worldwide intellectual property portfolio.
Sponsored Content Policy: News-Medical.net publishes articles and related content that may be derived from sources where we have existing commercial relationships, provided such content adds value to the core editorial ethos of News-Medical.Net which is to educate and inform site visitors interested in medical research, science, medical devices and treatments.