Sponsored Content by CYTENA GmbHReviewed by Maria OsipovaAug 1 2024
This article is based on a poster originally authored by Julia Scherzinger, Daniel Türk, and Fernando Aprile-Garcia from CYTENA GmbH.
There is constant pressure to reduce timelines in mammalian cell line development (CLD) for biotherapeutic protein production and gene and cell therapy. Demonstrating clonal derivation of generated cell lines is crucial for health authorities' approval. Single-cell dispensers and plate imagers have become essential in any CLD laboratory to meet these regulatory and process-oriented demands.
The UP.SIGHT 2nd Gen (CYTENA GmbH) is an all-in-one solution for generating monoclonal cell lines. This platform enables the complete workflow from single-cell cloning to the selection of high-producing clones, with comprehensive documentation for IND/BLA submissions.
This system assures over 97 % single-cell dispensing efficiency (SCDE) and over 99.99 % probability of clonal derivation (p(clonal)). Results demonstrate that the UP.SIGHT 2nd Gen facilitates fast and efficient CLD workflows with full documentation of clonal derivation.
- The UP.SIGHT 2nd Gen softly dispenses single cells in 384- and 96-well plates, ensuring clonal development.
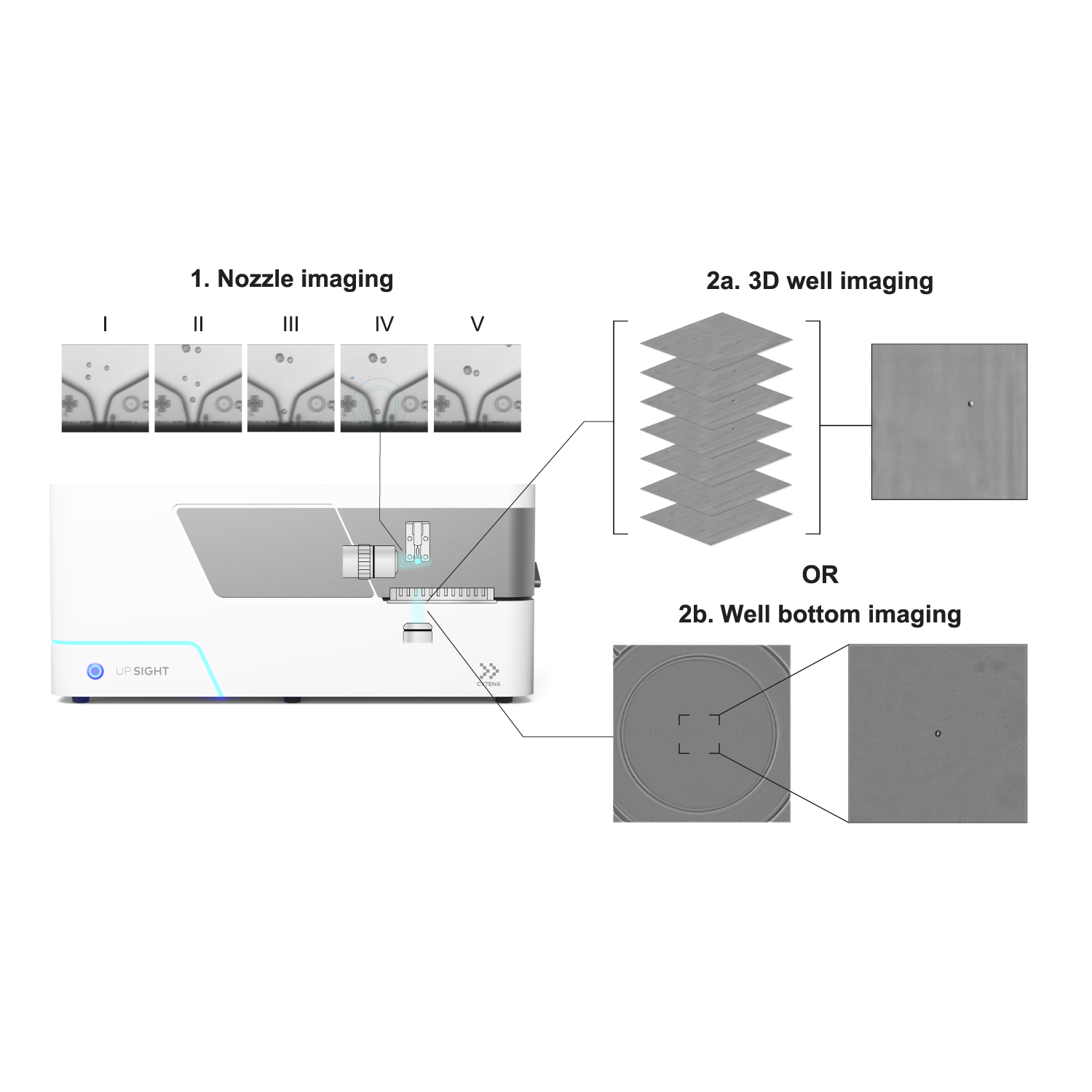
1. Nozzle imaging: Droplets containing one cell are dispensed. I, before single-cell dispensing; II, cell enters region of interest; III, cell is at the nozzle; IV, same image with cell detection overlay; V, after single-cell dispensing to verify that cell left nozzle. 2a. Day 0 3D well imaging: Generation of image stack to confirm single cell deposition. 2b. Day 0 well bottom imaging: Acquisition of single image once cell has settled to the bottom of the well to confirm single cell deposition. Image Credit: CYTENA GmbH
- The UP.SIGHT 2nd Gen has a single-cell dispensing efficiency of over 97 %.
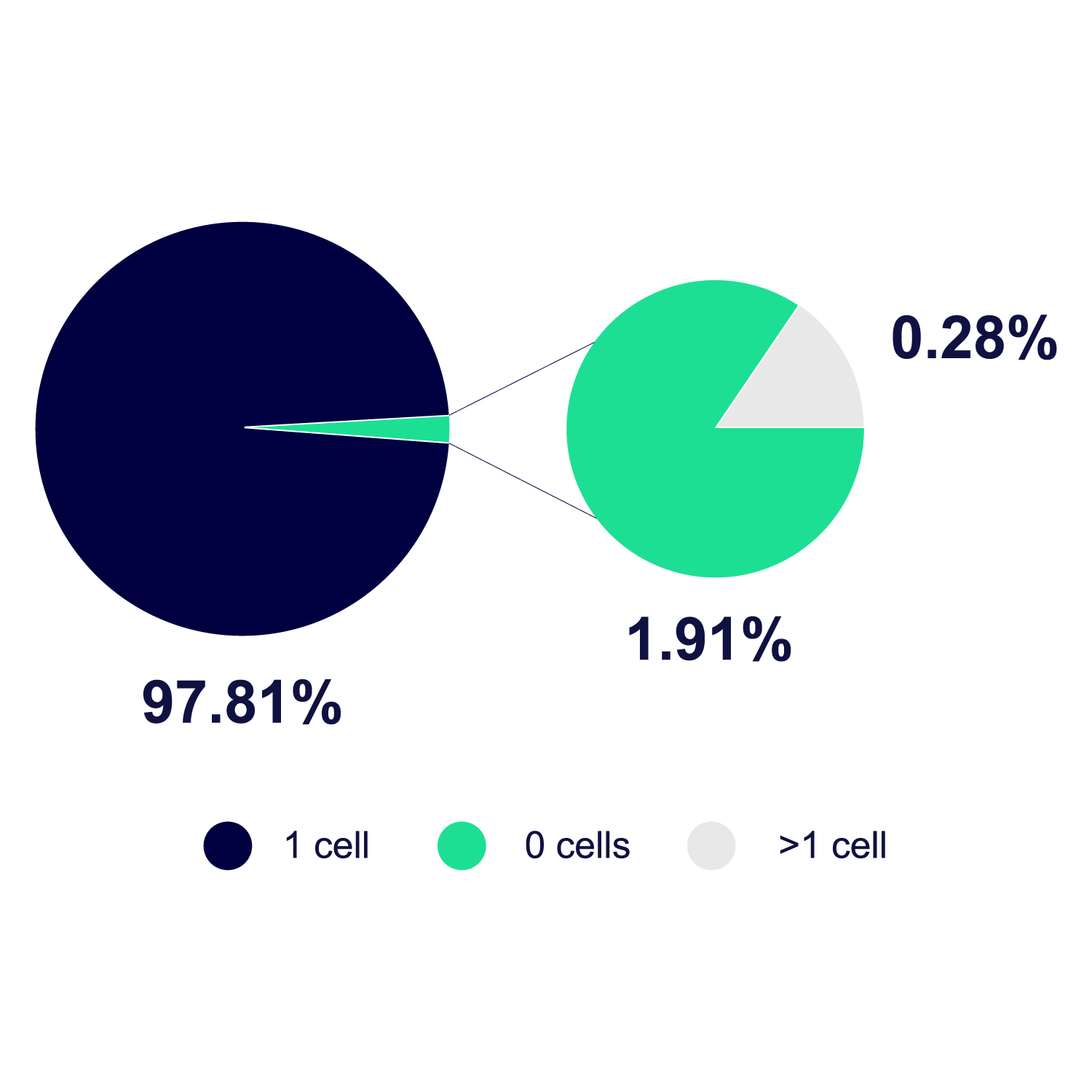
CHO-GFP cells were dispensed in 3 independent experiments with 3 UP.SIGHT 2nd Gens each. In total, 45 384-well plates (17280 wells) were analyzed. The number of cells deposited in each well was determined by well bottom imaging with a NYONE imager. Results were curated manually to correct algorithm-caused mistakes. Image Credit: CYTENA GmbH
- The probability of clonal derivation employing 3D well imaging on the UP.SIGHT 2nd Gen exceeds 99.99 %.
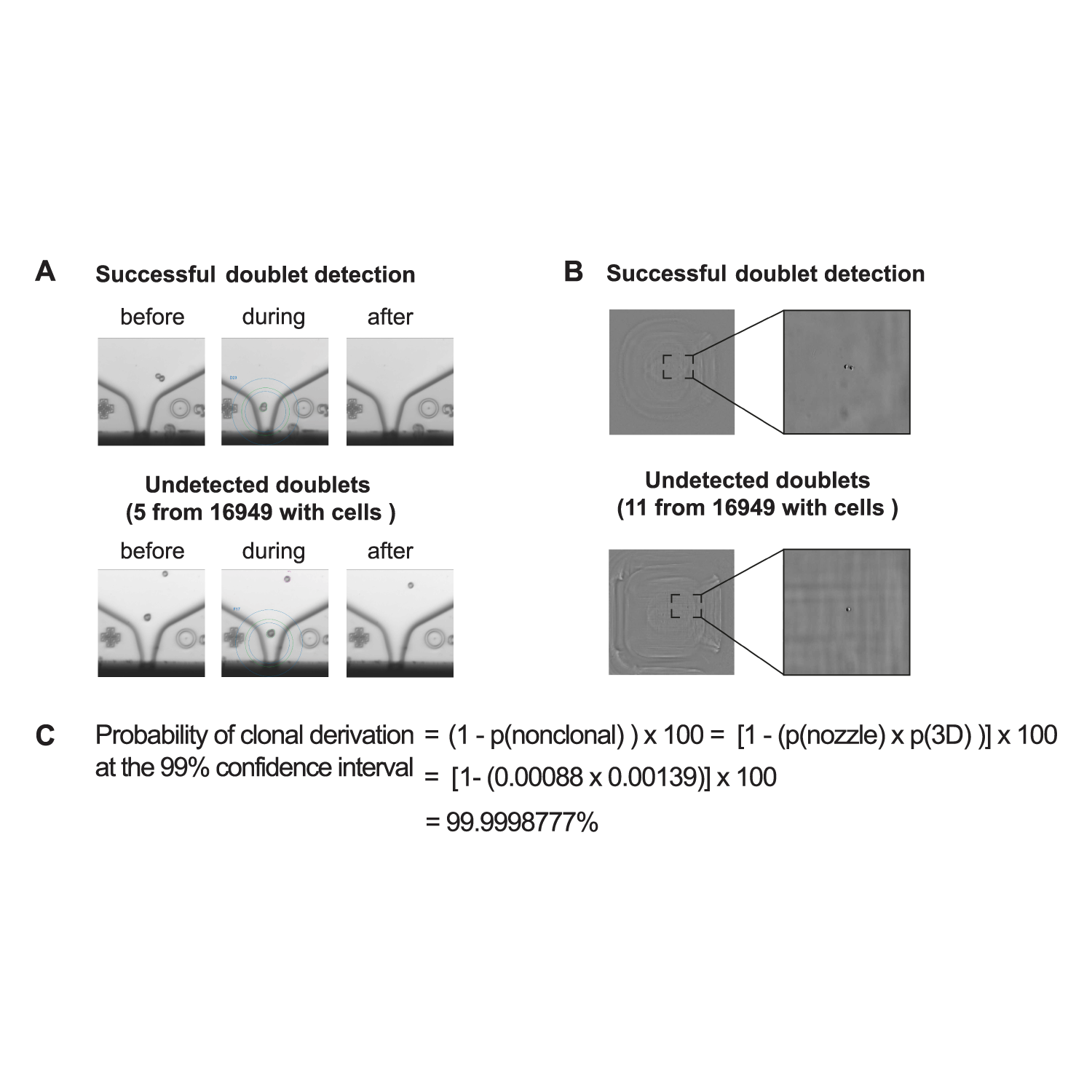
A) Nozzle images. Top: doublet detection. Bottom: no doublet detection; nozzle imaging system fails at detecting the doublet. This accounts for the nozzle system detection error. B) Individual stack images from 3D well imaging. Top: doublet detection. Bottom: no doublet detection; 3D well imaging system fails at detecting the doublet. This accounts for the 3D well imaging system detection error. C) A clone is of non-clonal origin if both imaging systems failed to detect a doublet. The error rate of each imaging system was statistically derived with the Wilson Method after image inspection and the resulting probability of clonal derivation was calculated as shown. Image Credit: CYTENA GmbH
- Well-bottom imaging monitors the growth of suspension and adherent cell lines using confluency assessment and cell counting.
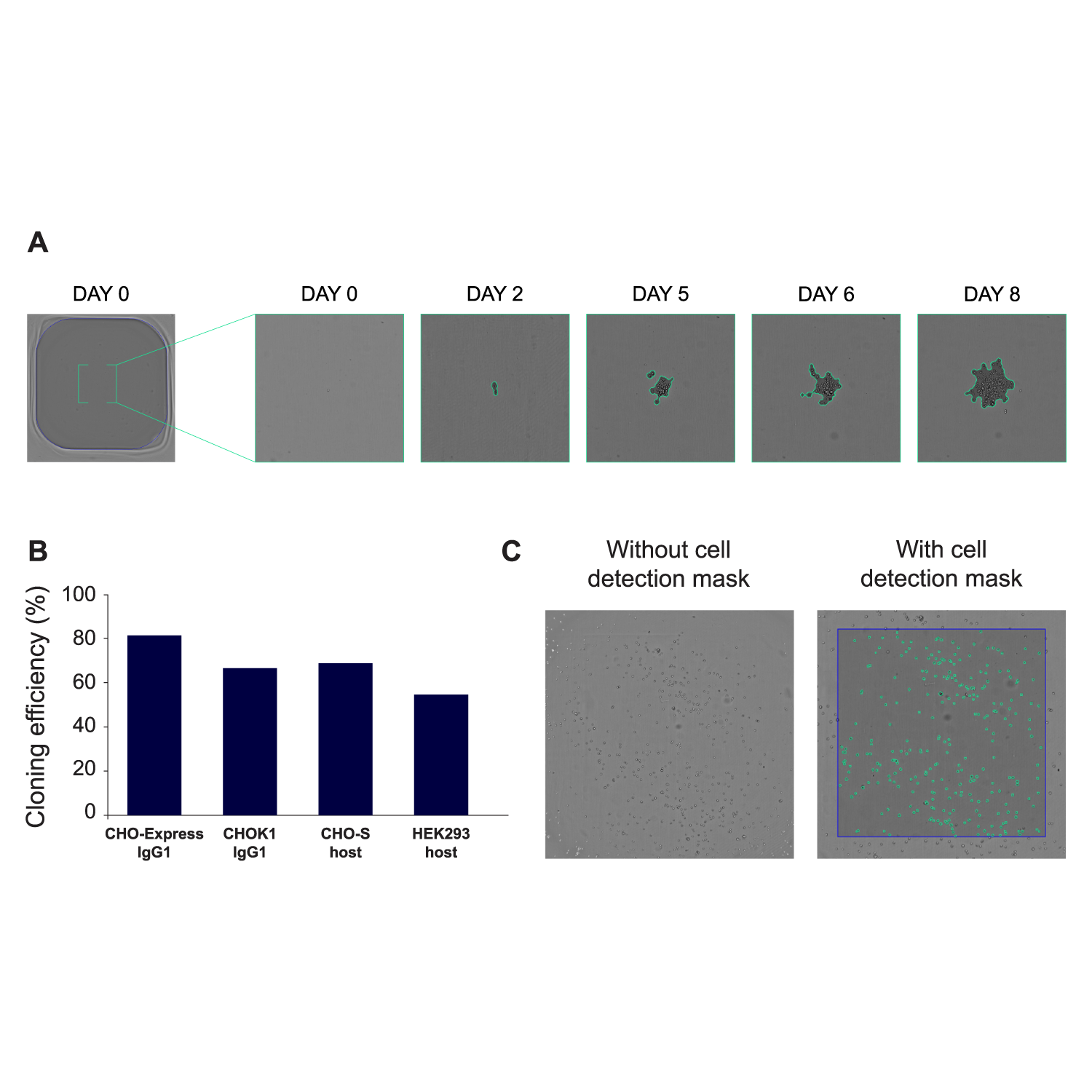
A) UP.SIGHT 2nd Gen well bottom images at different timepoints after CHO single cell dispensing. B) Typical cloning efficiencies after medium optimization for several suspension cell lines. Cloning efficiency values expressed as percentage of wells occupied by colonies on 384-well plates after 14 days of cloning. N=3-5, error bars: s.e.m. C) Representative cell count image with and without cell detection mask (2.5x10⁴ cells/ml). All image analysis performed with C.STUDIO. Image Credit: CYTENA GmbH
- F.QUANT-based mAb titer assessment for clone selection.
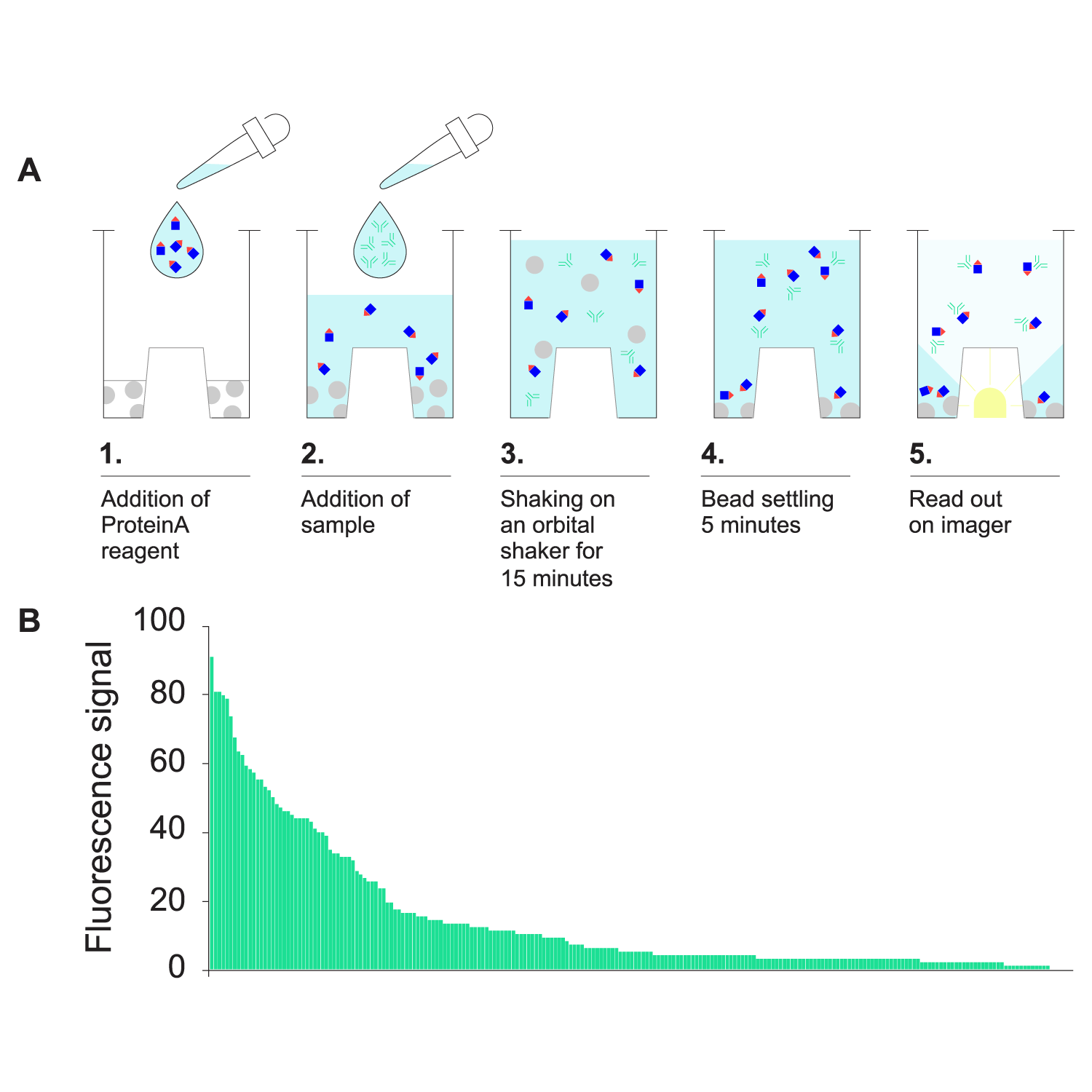
A) Schematic protocol for F.QUANT Fc Titer Assay. B) A minipool of IgG1-expressing CHO cells was dispensed into 384-well plates using the UP.SIGHT 2nd Gen. On day 10, plates were imaged and confluency determined with C.STUDIO. The top 25% clones (227 clones) were picked into 96-well plates. 3 days later, 5 μl supernatant was used for F.QUANT-based titer measurement. Ranked relative titer values are shown. Image Credit: CYTENA GmbH
- C.STUDIO: Analysis, clone tracking, and reporting.
- Select your best clones based on all UP.SIGHT 2nd Gen-derived data and even import custom data.
- Generation of clonality reports to prove clonal derivation of producer cell lines.
- Confluency measurement to track cell growth over time.
- Determination of cell counts.
- Comprehensive mAb monitoring for high-producing clone identification.
- Software-guided clone transfer: C.STUDIO creates clone transfer lists to aid in manual pipetting.
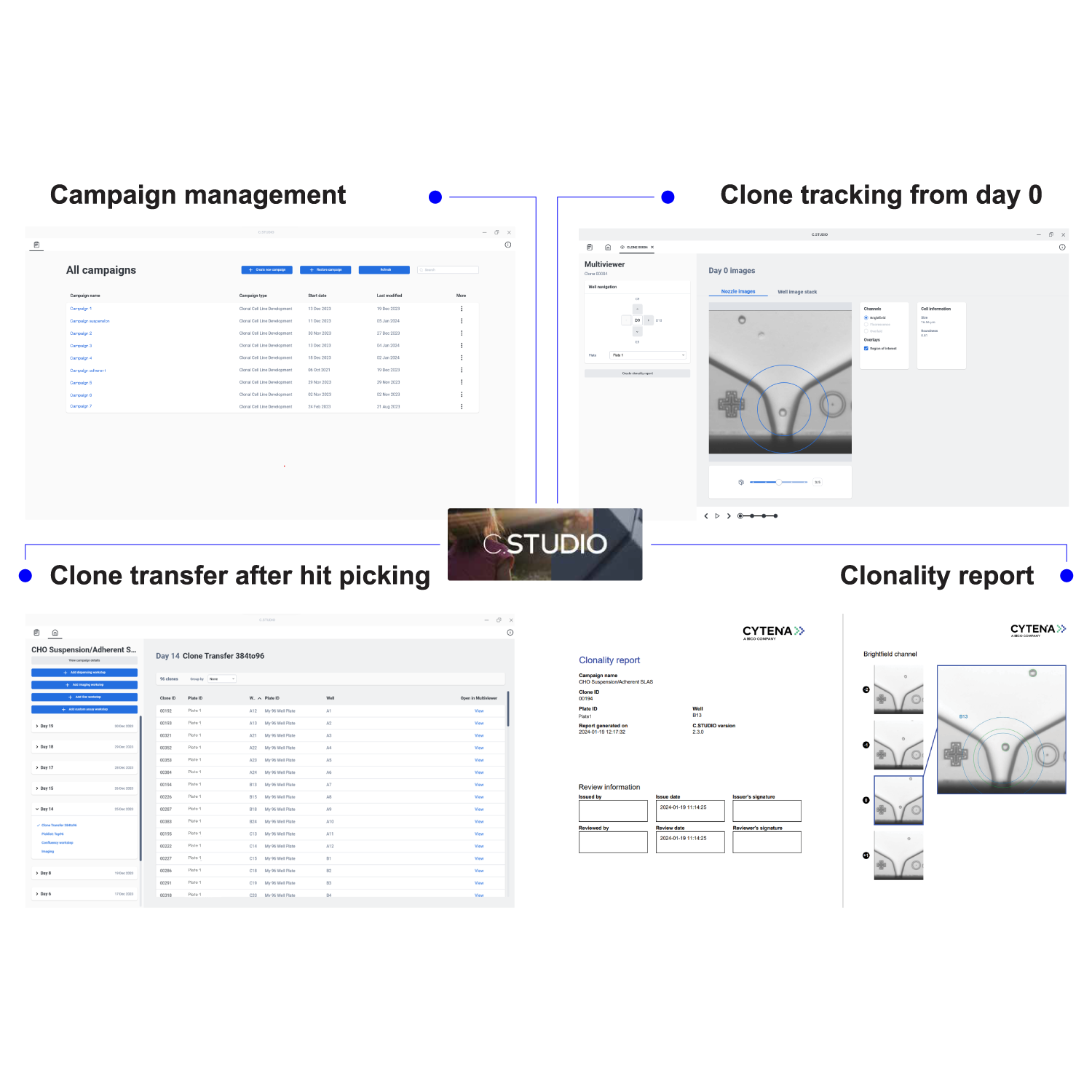 Image Credit: CYTENA GmbH
Image Credit: CYTENA GmbH
Conclusions
- The UP.SIGHT 2nd Gen is a gentle single-cell dispenser with high efficiency and clonal derivation assurance.
- Plate imaging and C.STUDIO image analysis allow for colony tracking and clone characterization over time.
- C.STUDIO selects high-producing clones based on UP.SIGHT 2nd Gen-derived titer measurements.
- The same instrument covers all phases, including single cell dispensing, clonality confirmation, and colony tracking. This should result in a faster and more effective cloning technique, as well as greater documentation for increased quality of the final biological output.
Download the Poster
About CYTENA GmbH
CYTENA is a multiple award-winning biotechnology company providing cutting-edge single-cell dispensers, microplate washers, liquid handlers, and live-cell analyzers to scientists and researchers at the world’s top institutions. Founded in 2014 and headquartered in Freiburg, Germany, CYTENA became part of the BICO bio-convergence revolution in 2019, joining 1000+ colleagues and 13 companies offering a portfolio of technologies, products, and services to create the future of medicine.
Sponsored Content Policy: News-Medical.net publishes articles and related content that may be derived from sources where we have existing commercial relationships, provided such content adds value to the core editorial ethos of News-Medical.Net which is to educate and inform site visitors interested in medical research, science, medical devices and treatments.