STEAP1 (Six-transmembrane epithelial antigen of the prostate 1), which was first found in prostate cancer, is a member of the STEAP protein family that is largely expressed in prostate epithelial cells and diverse malignant tumor cells.
Increasing research has shown that STEAP1 is highly expressed in a variety of solid tumors, including prostate cancer, bladder cancer, colorectal cancer, breast cancer, and non-small cell lung cancer, and is associated with tumor progression and a poor prognosis, making it a potential tumor target.
Structure and function of STEAP 1
STEAP1 is a six-transmembrane protein that exists on the cell membrane surface. The STEAP family also comprises STEAP1B (a shortened homolog of STEAP1), STEAP2, STEAP3, and STEAP4, all of which play a role in metal ion metabolism, particularly iron and copper reduction.
STEAP1 lacks the N-terminal NADPH oxidoreductase (FNO) domain, preventing it from performing metal reduction processes on its own. Instead, it may indirectly participate in metal metabolism by interacting with STEAP2 or STEAP4.
In addition, STEAP1's partial colocalization with transferrin (Tf) and transferrin receptor 1 (TfR1) implies a potential role in iron metabolism. Because STEAP1 is typically found at cell junctions on the cell membrane, it may act as a channel or transporter in intercellular signaling.
Although the precise functions of STEAP1 are unknown, its potential roles in metal metabolism and cell communication make it a valuable research target.
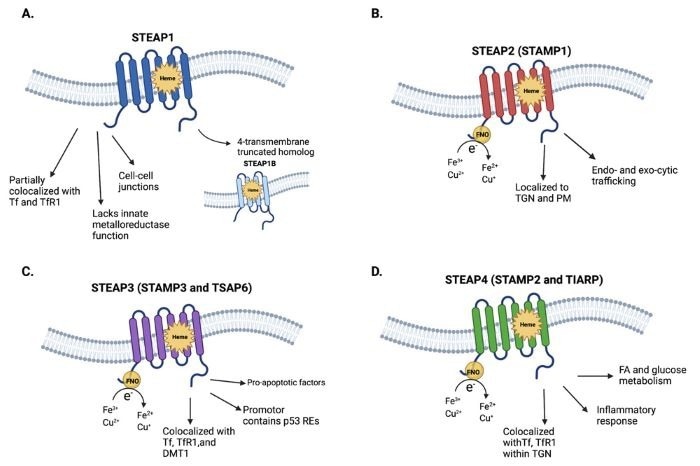
The structures and physiological characteristics of STEAP1-4. Image Credit: ACROBiosystems
The dual roles of STEAP1 in different cancers
STEAP1 plays two roles in distinct malignancies. In some tumors, it stimulates tumor formation; in others, it may suppress tumor growth.
Promote tumor progression
- Colorectal cancer: STEAP1 is associated with reactive oxygen species (ROS) levels and affects ROS levels by regulating the NRF2 pathway. Knockdown of STEAP1 can reduce ROS production and promote cancer cell apoptosis.
- Gastric cancer: Upregulation of STEAP1 is closely associated with tumor proliferation, migration, and peritoneal metastasis. Knockdown of STEAP1 can effectively reduce these malignant processes.
- Liver cancer: Knockdown of STEAP1 can inhibit the expression of the oncogene c-Myc, causing tumor cells to arrest in the G1 phase and significantly inhibiting their proliferation.
- Lung cancer: Through the JAK2/STAT3 signaling pathway, STEAP1 promotes cell migration and angiogenesis. Knockout of STEAP1 can significantly inhibit the proliferation, migration, and invasion of tumor cells.
- Ovarian cancer: STEAP1 can promote epithelial-mesenchymal transition (EMT), accelerate cell invasion and metastasis. Knockout of STEAP1 can inhibit cell proliferation and migration, and promote cell apoptosis.
- Prostate cancer: STEAP1 is highly expressed in prostate cancer, especially in metastatic castration-resistant prostate cancer (mCRPC), and its expression level is closely related to disease progression. Knockdown of STEAP1 can induce cancer cell apoptosis and reduce their proliferation.
Inhibit tumor progression
- Breast cancer: STEAP1 inhibits breast cancer cells and promotes their invasiveness, leading to overexpression of EMT-related genes.
- Endometrial cancer: STEAP1 inhibits endometrial cancer. Downregulation of STEAP1 can speed up cancer cell proliferation, migration, invasion, and EMT progression.
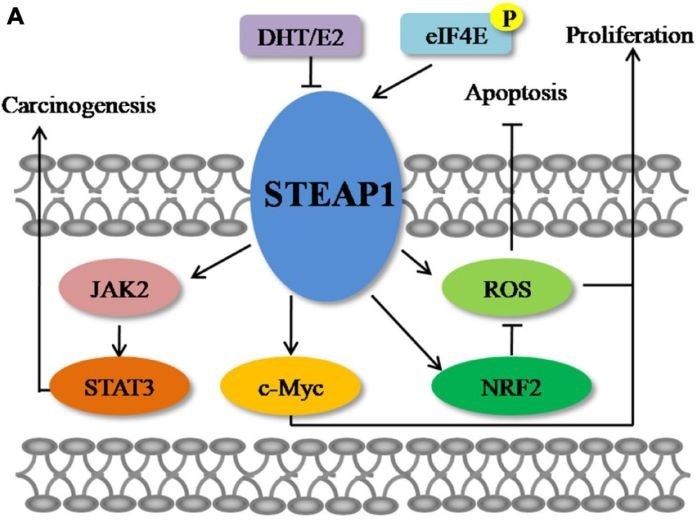
The molecular mechanisms of STEAP1 in cancer. Image Credit: ACROBiosystems
Targeted therapy for STEAP1
Antibody therapy
Antibody-Drug Conjugates (ADCs) are currently a key strategy for targeting STEAP1. ABBV-969, an ADC that targets STEAP1, is now in Phase I clinical trials, primarily for the treatment of mCRPC.
The purpose of this study is to assess the safety, pharmacokinetics, and preliminary efficacy of ABBV-969, as well as to determine the appropriate therapeutic dose via dose escalation.
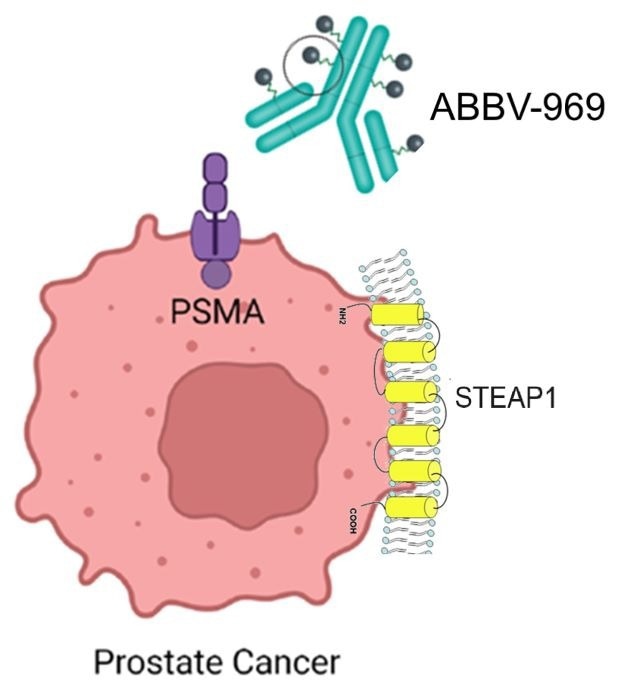
Mechanism of ABBV-969. Image Credit: ACROBiosystems
In addition, bispecific T-cell engager (TCE) therapy that targets STEAP1 is emerging as a promising treatment option.
AMG 509 is a TCE medication that targets both STEAP1 on tumor cells and CD3 on T-cells, promoting T-cell activation and killing tumor cells with strong STEAP1 expression. Early clinical trials have demonstrated that AMG 509 has promising potential as an immunotherapy for mCRPC.
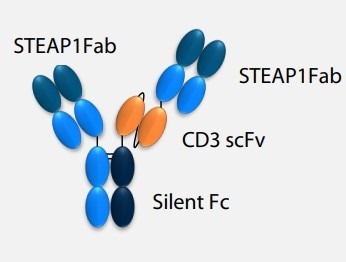
Structure of AMG 509. Image Credit: ACROBiosystems
CAR-T therapy
STEAP1 CAR-T is a modified CAR-T cell that targets prostate cancers, whereas Enzalutamide suppresses cancer cell proliferation by blocking androgen action.
On January 9th, 2024, Fred Hutchinson Cancer Research Center and PromiCell Therapeutics launched Phase I/II clinical research in the United States to assess the efficacy of combining STEAP1 CAR-T with Enzalutamide in the treatment of metastatic CRPC.
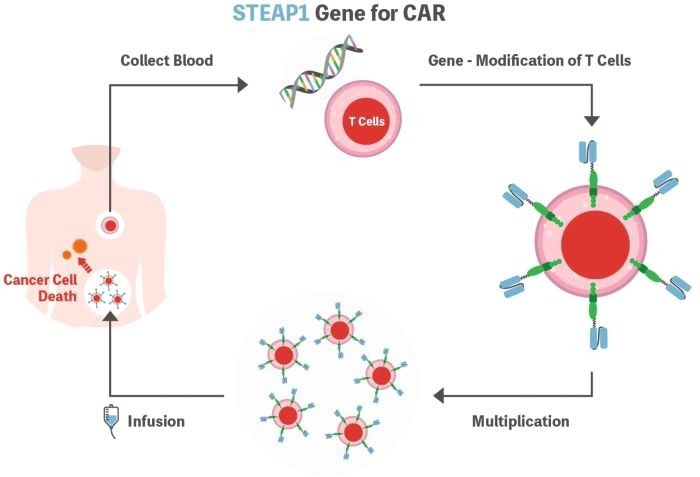
Illustration of STEAP1 CAR-T Cell. Image Credit: ACROBiosystems
mRNA vaccination therapy
STEAP1 has also been identified as a potential target for cancer vaccines. Ahvaz Jundishapur University of Medical Sciences is in the process of creating an mRNA vaccine for prostate cancer that uses an RNA-liposome delivery method for intravenous injection.
This potential vaccine targets three antigens linked to prostate cancer: PSMA, STEAP1, and PAP. By delivering these antigen modules, it activates the patient's immune system, allowing T-cells to recognize and attack cancer cells, resulting in an anti-tumor impact.
The vaccine is now undergoing Phase I clinical trials, which are primarily designed to test its safety and preliminary efficacy. It is hoped that this vaccine will provide a new immunotherapy option for prostate cancer patients.
Research and development pipeline of STEAP1. Source: global data
| Drug Name |
Company Name |
Indication |
Development Stage |
Target |
| ABBV-969 |
AbbVie Inc |
Metastatic Castration-Resistant Prostate Cancer (mCRPC) |
Phase I |
PSMA;
STEAP1 |
| ADRX-0405 |
Adcentrx Therapeutics Inc |
Gastric Cancer; Metastatic Castration-Resistant Prostate Cancer (mCRPC); Non-Small Cell Lung Cancer; Solid Tumor |
Phase I |
STEAP1; Topoisomerase |
| Vaccine to Target PSMA, STEAP1 and PAP for Prostate Cancer |
Ahvaz Jundishapur University of |
Prostate
Cancer |
Phase I |
PSMA; STEAP1;
PAP |
| Xaluritamig |
Amgen Inc |
Metastatic Castration-Resistant Prostate Cancer (mCRPC) |
Phase I |
CD3;
STEAP1 |
Anti-STEAP1 CAR
T-Cells |
Fred Hutchinson Cancer Research Center |
Castration-Resistant Prostate Cancer (CRPC) |
Preclinical |
STEAP1 |
| DXC-008 |
Hangzhou DAC Biotech Co Ltd |
Solid Tumor |
Preclinical |
STEAP1;
Tubulin |
| HLX-80 |
Shanghai Henlius Biotech Inc |
Castration-Resistant Prostate Cancer (CRPC) |
Preclinical |
STEAP1 |
| NTX-470 |
Nutcracker Therapeutics Inc |
Metastatic Castration-Resistant Prostate Cancer (mCRPC) |
Preclinical |
PSMA;
STEAP1 |
| STEAP1 |
Angeles Therapeutics Inc |
Unspecified |
Preclinical |
STEAP1 |
STEAP1 CAR
T Cells |
University of Oslo |
Metastatic Prostate Cancer |
Preclinical |
STEAP1 |
| STEAP1 x CD28 |
Xencor Inc |
Solid Tumor |
Preclinical |
STEAP1;
CD28 |
| CV-9103 |
Curevac NV |
Metastatic Castration-Resistant Prostate Cancer (mCRPC) |
Inactive |
PSMA; STEAP1;
PSA; PSCA |
| CV-9104 |
Curevac NV |
Metastatic Castration-Resistant Prostate Cancer (mCRPC) |
Inactive |
PSMA; STEAP1; MUC1; PSA; PSCA; PAP |
| Vandortuzumab Vedotin |
Genentech
USA Inc |
Metastatic Castration-Resistant Prostate Cancer (mCRPC) |
Discontinued |
STEAP1;
Tubulin |
ACROBiosystems successfully created full-length STEAP1 protein expressed in the HEK293 system using FLAG, a multi-pass transmembrane protein technology platform.
These products have natural conformation and full epitopes and have been verified by ELISA/SPR to display significant biological activity, making them suitable for the development of STEAP1-targeted medicines.

Image Credit: ACROBiosystems
References
- Xu, M., et al. (2022). STEAP1–4 (Six-Transmembrane Epithelial Antigen of the Prostate 1–4) and Their Clinical Implications for Prostate Cancer. Cancers, [online] 14(16), p.4034. https://doi.org/10.3390/cancers14164034.
- Chen, W.-J., et al. (2021). Regulatory Roles of Six-Transmembrane Epithelial Antigen of the Prostate Family Members in the Occurrence and Development of Malignant Tumors. Frontiers in cell and developmental biology, 9. https://doi.org/10.3389/fcell.2021.752426.
- Nakamura, H., Yohei Arihara and Takada, K. (2023). Targeting STEAP1 as an anticancer strategy. Frontiers in Oncology, 13. https://doi.org/10.3389/fonc.2023.1285661.
- Berger, R., et al. (2024). 1660TiP First-in-human study of ABBV-969, a dual variable antibody-drug conjugate (ADC), in patients (pts) with metastatic castration-resistant prostate cancer (mCRPC). Annals of Oncology, 35, pp.S1000–S1001. https://doi.org/10.1016/j.annonc.2024.08.1741.
- Wm. Kevin Kelly, et al. (2023). Xaluritamig, a STEAP1 × CD3 XmAb 2+1 Immune Therapy for Metastatic Castration-Resistant Prostate Cancer: Results from Dose Exploration in a First-in-Human Study. Cancer Discovery, pp.OF1–OF14. https://doi.org/10.1158/2159-8290.cd-23-0964.
About ACROBiosystems
ACROBiosystems is a cornerstone enterprise of the pharmaceutical and biotechnology industries. Their mission is to help overcome challenges with innovative tools and solutions from discovery to the clinic. They supply life science tools designed to be used in discovery research and scalable to the clinical phase and beyond. By consistently adapting to new regulatory challenges and guidelines, ACROBiosystems delivers solutions, whether it comes through recombinant proteins, antibodies, assay kits, GMP-grade reagents, or custom services. ACROBiosystems empower scientists and engineers dedicated towards innovation to simplify and accelerate the development of new, better, and more affordable medicine.
Sponsored Content Policy: News-Medical.net publishes articles and related content that may be derived from sources where we have existing commercial relationships, provided such content adds value to the core editorial ethos of News-Medical.Net which is to educate and inform site visitors interested in medical research, science, medical devices and treatments.