The need for viral vectors and plasmid DNA development is growing in parallel with the increase in demand for cell and gene therapy. Adeno-associated viruses (AAV), lentiviruses, retroviruses, herpes viruses, adenoviruses and other viral vectors are used in gene and cell treatments to transfer genes.
As infection with the vector is not harmful and AAV cannot reproduce on its own, AAV-mediated gene therapy is gaining the most attention among them.
The AAV viral vectors, as well as messenger RNAs derived from plasmid DNA templates, are crucial steps in the production and delivery of the chimeric antigen receptor (CAR) to T-cells, allowing CAR-T treatments to be developed.
Accurately measuring viral titer in gene therapies
As viral particles are created in live cells and then purified, controlling the concentration of possible treatment is tricky. In addition, host cell DNA, mycoplasma, and other pollutants can contaminate the process of creating and purifying viral vectors. To guarantee proper formulation, viral titer must be evaluated properly, precisely and consistently.
To assure product quality and safety, harmful adventitious agents (virus, bacteria and fungi) and contaminated DNA must be recognized and filtered out with maximum sensitivity.
While real-time PCR is still the gold standard for quantifying therapeutic viral vectors like AAV used in cell and gene treatments, digital PCR (dPCR) offers considerable benefits for quantifying target DNA molecules in a variety of analytical tests required for viral vector production and characterization.
Unlike real-time PCR, dPCR may yield an absolute nucleic acid count, allowing for the exact measurement of AAV vectors, bacterial contamination and residual host cell DNA. There is no need for a reference standard, which improves precision and eliminates a source of variance.
The varied architectures of the relevant DNA species - helical, supercoiled, linear DNA or viral particles - contribute to the variability in the standard curve used for real-time PCR. dPCR is also less susceptible to impurities that impact amplification, such as those seen in AAV-mediated gene therapy development solutions.
Absolute Q Viral Titer dPCR assays
The Absolute Q Viral Titer dPCR assays from Applied Biosystems make viral vector quantification simple and accurate. To quantify concentration and assess quality for biopharma and gene therapy research, the assays may be run multiplexed or individually utilizing a customized assay with the target gene of interest.
Absolute Q dPCR tests are:
- Fast — Less hands-on time; when used with the QuantStudio Absolute Q Digital PCR System, results are available in just 90 minutes
- Simple — Optimized process for digital PCR instrument ease of use
- Dependable — Analyze data with confidence using confirmed assays that are supported by a performance guarantee*
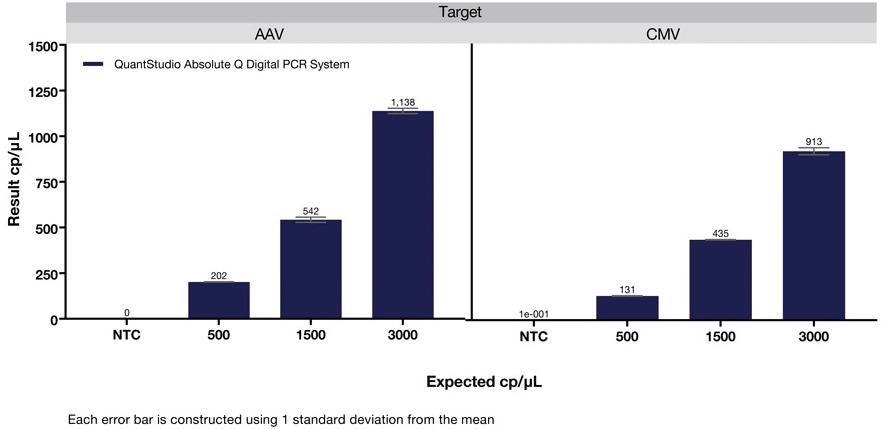
Figure 1. Quantification of AAV and CMV viral vector dilution series on the QuantStudio Absolute Q dPCR System. Image Credit: Thermo Fisher Scientific Applied Biosystems, qPCR
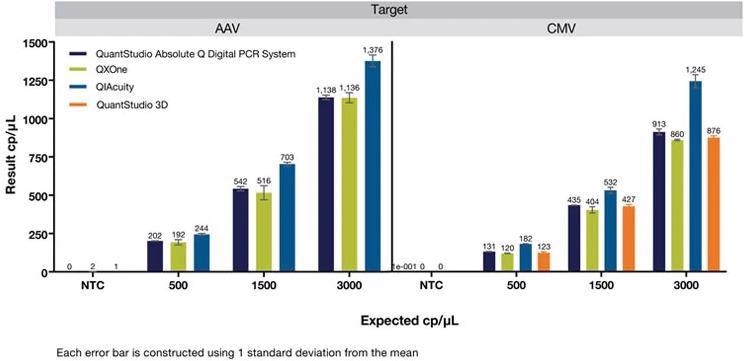
Figure 2. Quantification of AAV and CMV viral vector dilution series across multiple digital PCR platforms using the Absolute Q(TM) AAV and Absolute Q(TM) CMV dPCR Assays. Image Credit: Thermo Fisher Scientific Applied Biosystems, qPCR