A team of scientists from the United States has recently revealed that about 25% of people with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection develop renal complications and that the viral spike protein can be detected in the urine samples of these patients. The study is currently available on the medRxiv* preprint server.
The coronavirus disease 2019 (COVID-19) pandemic caused by SARS-CoV-2 has already affected more than 103 million people and claimed over 2.23 million lives worldwide. Although around 80% of COVID-19 patients remain asymptomatic or mildly symptomatic, a significant proportion of COVID-19 recovered patients have been found to suffer from long-term complications. The mechanism of SARS-CoV-2 infection is now well-established that interaction between the receptor-binding domain (RBD) of the viral spike protein and the host angiotensin-converting enzyme 2 (ACE2) is the initial step of viral entry. This step is followed by the priming of the spike protein by cellular proteases, leading to cell-cell fusion and the virus's entry into host cells.
Given its significant involvement in SARS-CoV-2 infection, screening the viral spike protein is crucial for monitoring active and recent COVID-19 patients. Besides lung epithelial cells, ACE2 is highly expressed in the epithelial cells of the renal, gastrointestinal, cardiovascular systems, suggesting that SARS-CoV-2 can potentially enter and infect multiple organs, including kidneys. Regarding the long-term impacts of SARS-CoV-2 infection in the renal system, some studies have shown the development of acute or chronic kidney disease in COVID-19 patients.
In the current study, the scientists have conducted an antigen-capture assay to detect SARS-CoV-2 spike protein in the urine samples obtained from clinically confirmed COVID-19 patients. They have also analyzed urine samples collected 3 – 5 years before the onset of the pandemic.
Study design
The analysis was conducted using 233 urine samples obtained from 132 participants (both adults and children), in addition to 20 pre-pandemic urine samples. Of all adult participants, 91 were COVID-19 positive and 15 were COVID-19 negative. Of all enrolled children, 12 were COVID-19 positive and 14 were negative. Pre-pandemic samples and COVID-19 negative samples were used as controls.
In addition to detecting the viral spike protein, the scientists measured the levels of electrolytes, creatinine, albumin, and cystatin C in the urine samples.
Important observations
The antigen capture assay used in the study involved capturing the viral antigen using anti-spike polyclonal antibody-mediated ELISA and detecting antigen-antibody complex using biotinylated antibodies.
Of 91 COVID-19 positive adults, 23 showed the presence of viral spike protein in the urine (25%). In contrast, none of the COVID-19 positive children showed the presence of spike protein in the urine. Interestingly, one COVID-19 negative child showed high levels of spike protein in the urine. According to the scientists, this child was infected with SARS-CoV-2 previously but still continued to shed viral protein in the urine.
Furthermore, no correlation was observed between the urinary presence of viral spike protein and gender, age, body mass index (BMI), and duration of hospital stay. Of all COVID-19 positive patients, less than 2% showed the presence of viral RNA in the urine, indicating that the presence of viral spike protein in the urine is not due to the presence of SARS-CoV-2-infected cells.
Regarding infiltrated proteins in the urine, all COVID-19 positive patients showed higher albumin and cystatin C concentrations in the urine than in COVID-19 negative participants. The highest level of urinary albumin was observed in patients who were detected with spike protein in the urine sample.
A urinary albumin level of 0.3 mg/mg urine creatinine is generally indicative of acute kidney infection. Considering this cut-off value, a significant correlation was observed between the concentration of spike protein in the urine and the elevated urine albumin/creatinine ratio. Furthermore, no significant correlation was observed between the spike protein levels in the urine and in the serum, indicating that a high serum level of viral spike protein is not responsible for its urinary infiltration.
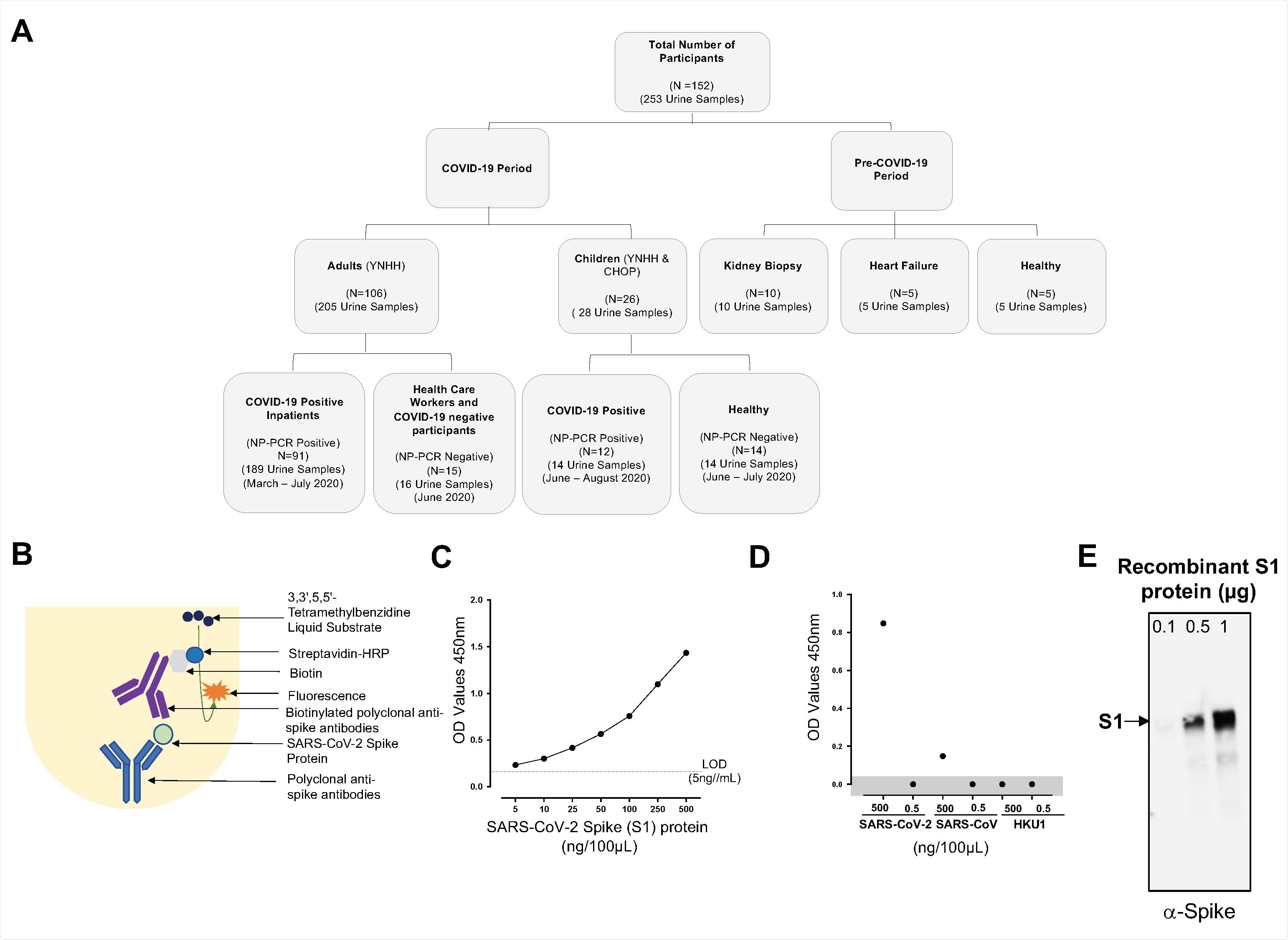
Consolidated summary of study population, assay chemistry, and sensitivity and specificity of the SARS-CoV-2 spike protein using capture ELISA. (A) Flowchart describing the study population. Samples used in this study were collected both before and during the COVID-19 pandemic. (B) Schematic representative of the Capture ELISA assay chemistry. (C) Representative standard curve generated using 5μg/mL SARS-CoV-2 polyclonal anti-spike antibodies. (D) Assay to define the specificity of the SARS-CoV-2 Capture ELISA. Two different concentrations (5μg/mL and 5ng/mL) of different coronavirus infecting humans (SARS-CoV-2, SARS-CoV and HCoV-HKU1) were assessed to determine the specificity of the Polyclonal anti-spike SARS-CoV-2 antibodies. Data points in the shaded area are below the limit of detection. (E) Sensitivity of the polyclonal antibodies to detect SARS-CoV-2 spike S1 protein using Western blot. SARS-CoV-2 in three different concentration was measured 0.1μg, 0.5μg and 1μg.
Study significance
The study indicates that shedding of viral spike protein occurs in about 25% of confirmed COVID-19 patients. Notably, the shedding is not due to the presence of infected cells in the urine or high levels of viral protein in the serum. Moreover, the study emphasizes that acute/chronic kidney complications might be regarded as long-term outcomes of SARS-CoV-2 infection.
Source

 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources