A team of scientists from the United States has recently compared the immune response elicited by natural severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection and coronavirus disease 2019 (COVID-19) vaccination. Their findings reveal that, unlike vaccination, natural SARS-CoV-2 infection is associated with a robust interferon response together with an induction of cytotoxic gene expression in peripheral blood lymphocytes. The study is currently available on the medRxiv* preprint server.
Since its emergence in December 2019, SARS-CoV-2 infection has been found to associate with highly heterogeneous clinical presentation, ranging from mild illness to acute respiratory distress, severe cardiovascular complications, multiorgan failure, and death.
Regarding the immune response to SARS-CoV-2, studies have shown that a significant proportion of COVID-19 patients present with lymphopenia, which is defined as a lower than normal level of blood lymphocytes. Moreover, alterations in the level and activity of specific immune cells, such as activated CD8+ T cells, natural killer cells, plasma cells, and monocytes, have been found to influence the clinical consequences of COVID-19 patients. In COVID-19 recovered patients, robust memory B, and T cell responses together with neutralizing antibody response has been observed.
Because of its significant involvement in the virus entry into host cells, the spike glycoprotein on the SARS-CoV-2 envelop is considered to be the most immunogenic component. This makes the protein the primary target for vaccine development. Studies investigating anti-COVID-19 immunity have shown that both natural infection and vaccination induce a strong protective immune response against the spike protein.
In the current study, the scientists have performed multimodal, single-cell sequencing to characterize and compare the host immune responses induced by natural SARS-CoV-2 infection and COVID-19 vaccination.
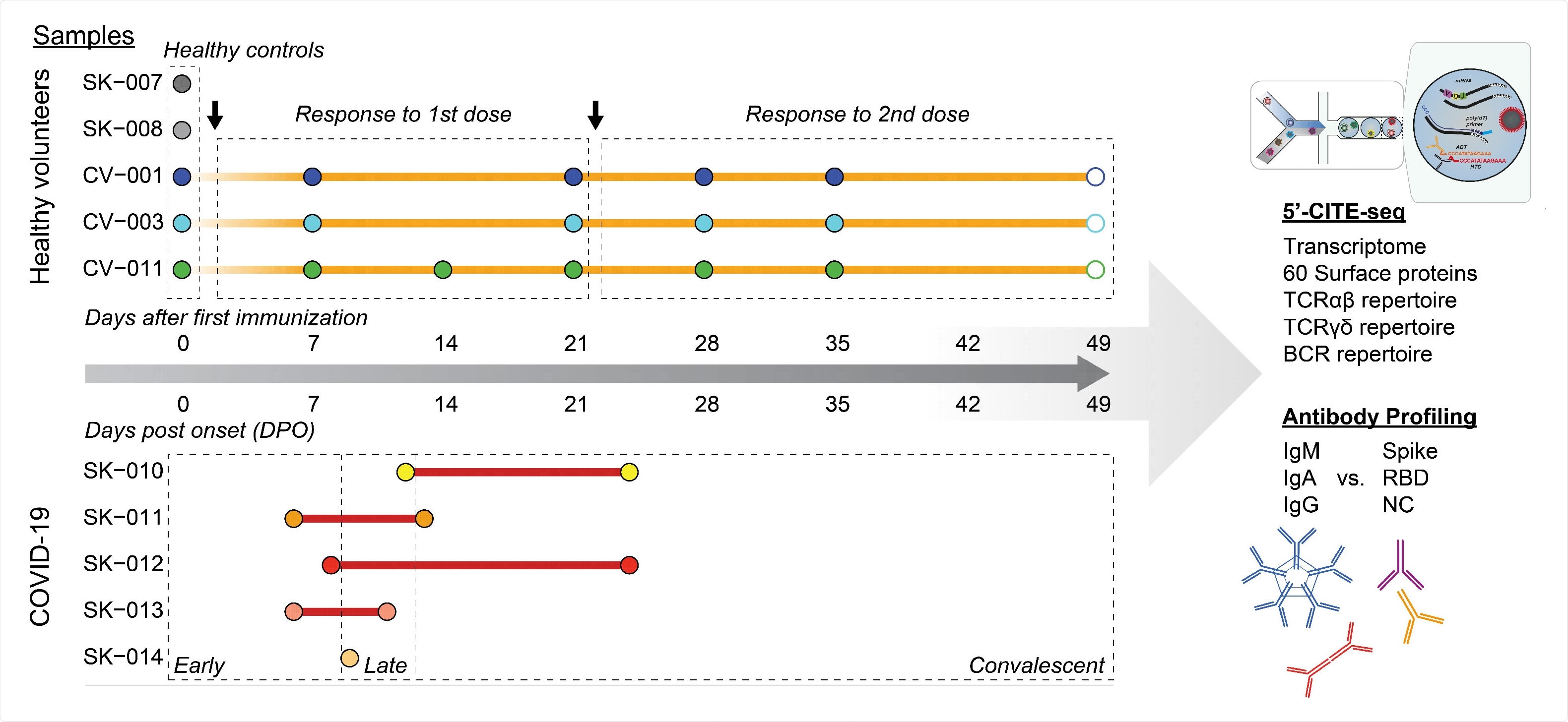
Study design
For single-cell ribonucleic acid (RNA sequencing, the scientists collected blood samples from five confirmed COVID-19 patients, three BNT162b2-vaccinated individuals, and two individuals without infection or vaccination. They performed a multiplex bead-binding assay to measure the titers of anti-SARS-CoV-2 antibodies in COVID-19 patients and vaccinated individuals.
The BNT162b2 COVID-19 vaccine developed by Pfizer/BioNTech is a lipid nanoparticle-formulated messenger RNA (mRNA) vaccine that contains a prefusion-stabilized full-length spike protein as a vaccine antigen.
Important observations
By conducting sequencing analysis, the scientists characterized transcriptional profiles and surface marker phenotypes in peripheral blood immune cells isolated from COVID-19 patients and vaccinated individuals. The findings revealed a significant difference between immune responses induced by natural infection and vaccination.
Specifically, the scientists observed that the frequency of hematopoietic stem and progenitor cells and key myeloid cells (monocytes and dendritic cells) in the blood increased significantly in COVID-19 patients. However, they did not observe any such alteration in immune cell subsets in vaccinated individuals. The immune response observed in COVID-19 patients highlights the urgent need for developing innate immune cells (neutrophils, basophils, eosinophils, monocytes, and dendritic cells) from myeloid progenitors.
By analyzing transcriptional signature in myeloid cells, the scientists observed that the expressions of genes associated with type I and type II interferon production increased significantly in COVID-19 patients but not in vaccinated individuals. These observations highlight the involvement of interferon signaling pathways in triggering inflammatory immune responses against viral infection.
Regarding humoral immunity, the scientists observed a comparable induction in anti-SARS-CoV-2 antibody titers among COVID-19 patients and vaccinated individuals. In contrast, they observed a significantly increased frequency of circulating plasmablasts in COVID-19 patients but not in vaccinated individuals. With further analysis, they observed that the plasmablasts from COVID-19 patients were highly enriched for genes associated with oxidative phosphorylation, type I and type II interferon responses, fatty acid metabolism, and mTORC1 signaling; whereas, the plasmablasts from vaccinated individuals displayed gene enrichment for TNF-NFkB signaling pathway.
By specifically analyzing the B cell clonal expansion, the scientists indicated that the increased interferon response during SARS-CoV-2 infection might have induced the differentiation of plasma cells in COVID-19 patients. In contrast, the vaccine seemed to trigger the expansion of circulating memory B cells.
Furthermore, they observed enrichment of activated T cells and natural killer cells with a high level of cytotoxic effector functions in COVID-19 patients. However, they could not detect such an immune signature in vaccinated individuals.
Study significance
The study findings identify a notable difference between the immune responses induced by natural SARS-CoV-2 infection and vaccination. While the vaccine-induced immune response is mainly associated with viral clearance and protective immunity, an immune reaction to SARS-CoV-2 significantly increases the risk of heightened inflammation and immunopathology.

 *Important notice: medRxiv publishes preliminary scientific reports that are not peer-reviewed and, therefore, should not be regarded as conclusive, guide clinical practice/health-related behavior, or treated as established information.
*Important notice: medRxiv publishes preliminary scientific reports that are not peer-reviewed and, therefore, should not be regarded as conclusive, guide clinical practice/health-related behavior, or treated as established information.
Journal reference:
- Preliminary scientific report.
Discrete immune response signature to SARS-CoV-2 mRNA vaccination versus infection, Ellie N Ivanova, Joseph C Devlin, Terild B Buus, Akiko Koide, Amber Cornelius, Marie I Samanovic, Alberto Herrera, Chenzhen Zhang, Ludovic Desvignes, Niels Odum, Robert Ulrich, Mark J Mulligan, Shohei Koide, Kelly V Ruggle, Ramin S Herati, Sergei B Koralov, medRxiv, 2021.04.20.21255677; doi: https://doi.org/10.1101/2021.04.20.21255677, https://www.medrxiv.org/content/10.1101/2021.04.20.21255677v1