Researchers in the United States have conducted a study demonstrating the real-world effectiveness of the Pfizer-BioNTech BNT162b2 and Moderna mRNA-1273 vaccines at preventing hospitalizations due to coronavirus disease 2019 (COVID-19).
The prospective observational case-control study showed that the vaccines were approximately 87% effective at preventing COVID-19 hospitalizations.
The study was conducted by the “Influenza and Other Viruses in the Acutely Ill (IVY) Network” at 18 geographically dispersed sites across the US during the early phase of the vaccination program.
“The vaccines were highly effective at preventing COVID-19 hospitalizations among adults in March through May 2021,” writes Wesley Self from Vanderbilt University Medical Center in Nashville and colleagues.
However, while vaccination was beneficial among patients with immunosuppression, it was less effective among this patient group and future work is needed to understand its efficacy among people with specific immunocompromising conditions, says the team.
A pre-print version of the research paper is available on the medRxiv* server, while the article undergoes peer review.

 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
More about vaccination roll-out
In December 2020, the Food and Drug Administration granted the emergency use of two messenger RNA- (mRNA) based vaccines developed by Pfizer-BioNTech and Moderna to protect against severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) – the agent responsible for the ongoing COVID-19 pandemic.
Widespread vaccine roll-out resulted in more than 60% of the US adult population receiving at least one vaccine dose by the end of May 2021.
Phase 3 clinical trials of these vaccines have previously demonstrated efficacies of 94 to 95% in preventing COVID-19 and an almost 100% efficacy in protecting against severe disease.
“However, these clinical trials had few cases of hospitalized COVID-19, and limited power to assess efficacy among persons with underlying illnesses who are at high risk for severe COVID-19,” writes Self and colleagues.
As vaccine coverage increases, there is a need to understand the preventive effect against COVID-19 in real-world settings across diverse populations, including immunocompromised individuals, say the researchers.
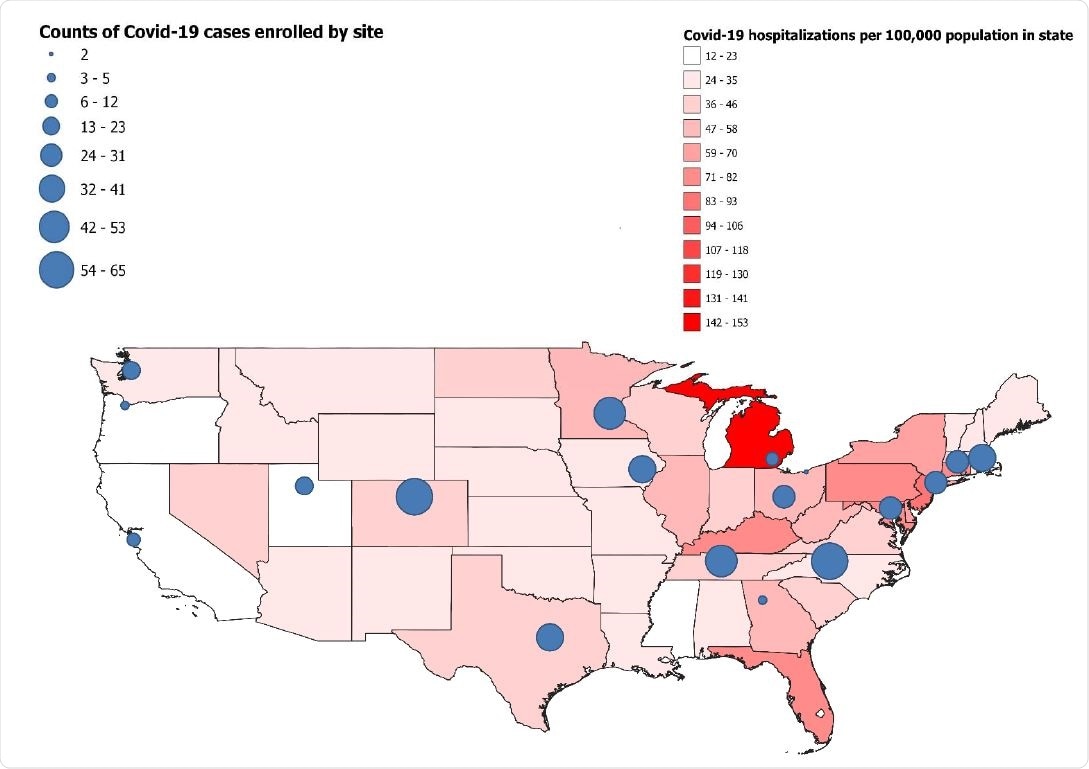
Map of continental United States with incidence of Covid-19 hospitalizations by state in April 2021 indicated by color (red). Participating sites are shown on the map with circles; the size of each circle represents the number of Covid-19 cases included from each site in this analysis -- IVY Network, United States, March–May 2021.
What did the researchers do?
In a prospective analysis of US adults hospitalized between March 11th and May 5th, 2021, the IVY Network compared the odds of prior immunization with the Pfizer-BioNTech or Moderna vaccine between cases hospitalized with COVID-19 and hospital-based controls without COVID-19.
The IVY Network is a collaborative funded by the Centers for Disease Control and Prevention (CDC) to monitor the effectiveness of SARS-CoV-2 vaccines at preventing COVID-19 hospitalizations among US adults. The study included 1,210 participants who were enrolled across 18 academic medical centers in 16 states.
Participants were considered fully vaccinated once 14 days had passed since they had received a second vaccine dose.
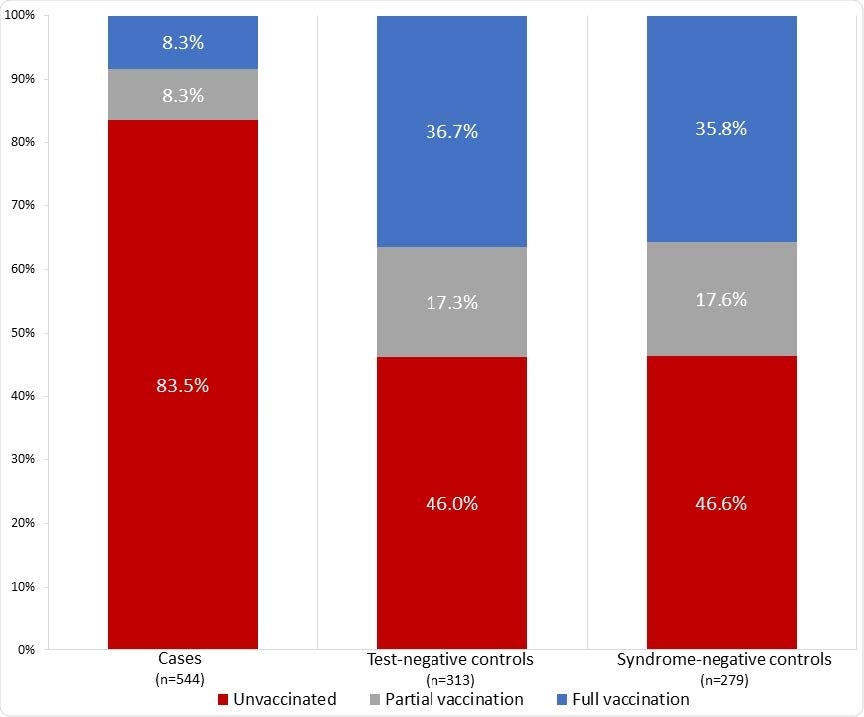
Vaccination status of case patients (N=544), test-negative controls (N=313), and syndromenegative controls (N=279) — IVY Network, United States, March–May 2021.
What did the study find?
Overall, the median age of participants was 58 years and 248 (20.6%) had an immunocompromising condition.
Among 590 cases with breakthrough COVID, only 45 (7.6%) were fully vaccinated, compared with 215 (34.7%) of 620 controls who did not have COVID-19.
Whole-genome sequencing of samples from 231 cases found that the most commonly identified SARS-CoV-2 lineage was the B.1.1.7 (alpha) variant.
The overall effectiveness of full vaccination at preventing COVID-19 hospitalization was 86.9%, with similar efficacies observed for the Pfizer-BioNTech (84.3%) vaccine and the Moderna (90.0%) vaccine.
The highest vaccine efficacy was observed among adults aged 18 to 49 years, at 97.3%.
Among adults aged 65 years or older, efficacy was similar between those aged 65 to 74 years (87.9%) and those aged 75 years or older (90.5%).
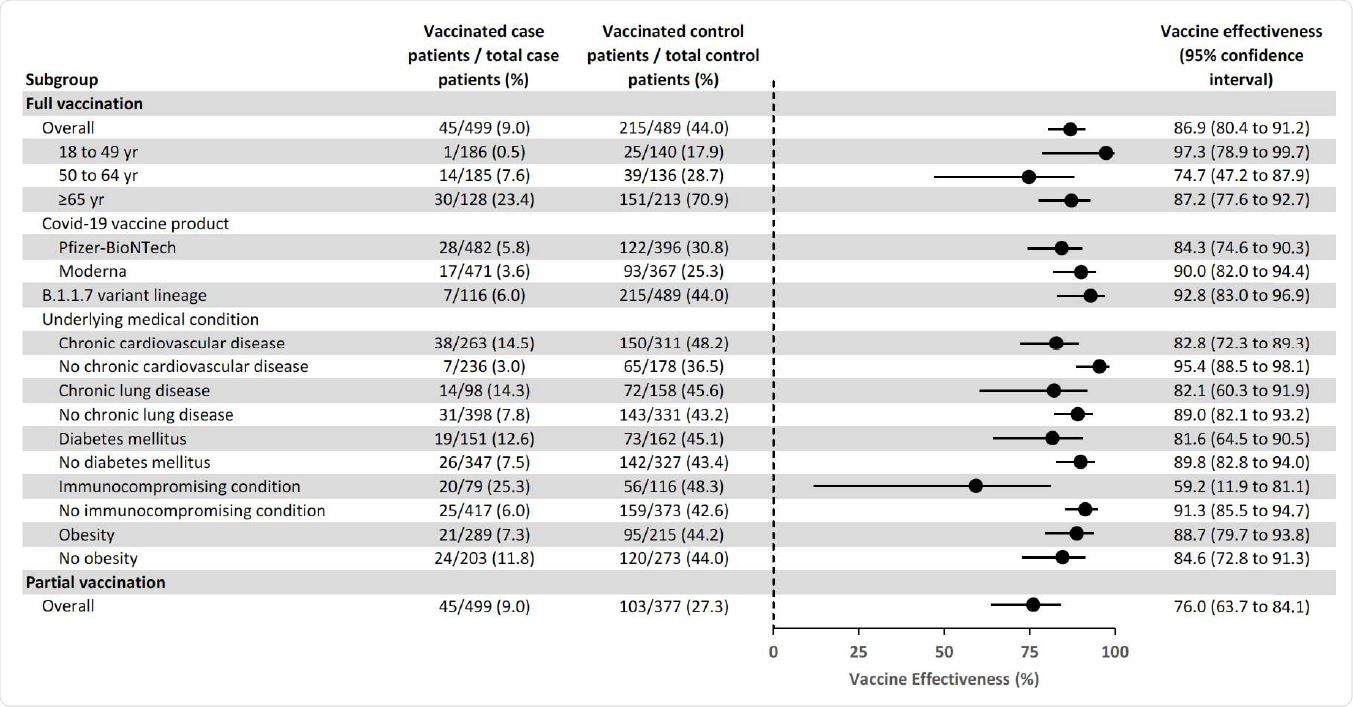
Vaccine effectiveness of SARS-CoV-2 mRNA vaccines for the prevention of Covid-19 hospitalizations overall and by subgroups — IVY Network, United States, March–May 2021.
Almost half of fully vaccinated cases had an immunocompromising condition
Vaccine efficacy was significantly reduced among patients with immunocompromising conditions, compared with individuals without immunosuppression, at 59.2% versus 91.3%.
Of the 45 fully vaccinated cases with breakthrough COVID-19, 20 (44.4%) had an immunocompromising condition. These conditions included an active solid organ or hematologic malignancy and prior solid organ transplant.
“A history of solid organ transplant and other immunocompromising conditions have been associated with reduced cell-mediated and humoral immune responses to SARS-CoV-2 vaccines,” say the researchers.
Vaccine efficacy was also lower among patients with underlying cardiovascular disease (82.8%), chronic lung disease (82.1%), and diabetes mellitus (81.6%) compared to patients without these conditions.
“Widespread vaccination can be expected to have a major beneficial impact”
“The findings of high vaccine effectiveness in the US adult population and across subgroups defined by age, demographics, and comorbidities suggest that the mRNA vaccines are broadly effective for the prevention of severe COVID-19, including in populations at high risk of severe illness,” writes Self and colleagues. “Widespread vaccination can be expected to have a major beneficial impact on COVID-19 hospitalizations and associated outcomes.”
However, while the vaccines appear to be beneficial to immunocompromised people, efficacy is lower in this population than among immunocompetent individuals, they add.
“Future work is needed to understand vaccine effectiveness among people with specific immunocompromising conditions and the durability of protection in this population to inform the need for booster vaccines and/or nonvaccine preventative interventions, such as mask use and social distancing,” advises the team.

 This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
This news article was a review of a preliminary scientific report that had not undergone peer-review at the time of publication. Since its initial publication, the scientific report has now been peer reviewed and accepted for publication in a Scientific Journal. Links to the preliminary and peer-reviewed reports are available in the Sources section at the bottom of this article. View Sources
Journal references:
- Preliminary scientific report.
Self W, et al. Effectiveness of SARS-CoV-2 mRNA Vaccines for Preventing Covid-19 Hospitalizations in the United States. medRxiv, 2021. doi: https://doi.org/10.1101/2021.07.08.21259776, https://www.medrxiv.org/content/10.1101/2021.07.08.21259776v1
- Peer reviewed and published scientific report.
Tenforde, Mark W, Manish M Patel, Adit A Ginde, David J Douin, H Keipp Talbot, Jonathan D Casey, Nicholas M Mohr, et al. 2021. “Effectiveness of SARS-CoV-2 MRNA Vaccines for Preventing Covid-19 Hospitalizations in the United States.” Clinical Infectious Diseases, August. https://doi.org/10.1093/cid/ciab687. https://academic.oup.com/cid/article/74/9/1515/6343399.