This article is based on a poster originally authored by Maryam Ahmadi, Elena Shvets, Frank Gesellchen, Xin Liu, Jessica Hall and Richard Hammond, which was presented at ELRIG Drug Discovery 2024 in affiliation with Fluidic Sciences and Sphere Bio.
This poster is being hosted on this website in its raw form, without modifications. It has not undergone peer review but has been reviewed to meet AZoNetwork's editorial quality standards. The information contained is for informational purposes only and should not be considered validated by independent peer assessment.

Although antibody therapeutics dominate drug development, prolonged workflows and increasing costs hinder their success during their discovery and development.1-3 Cyto-Mine® offers significant advantages in the number of cells that can be processed, cost per run, and the speed with which projects can be completed.
Fluidic Sciences and Sphere Bio has developed Cyto-Mine® Chroma, a new instrument that enables the use of more fluors, multiplexed assays, and a broader range of applications.
Early applications of the Cyto-Mine® Chroma platform will enable cell isolation based on cell surface markers, viability, and titer of secreted proteins. Re-injection-based workflows facilitate complex assays like combining more than one cell type in a single droplet (crucial for binding assessment, cytotoxic assays, and advancing antibody development).
Cyto-Mine®

Figure 1. The Cyto-Mine® platform. Image Credit: Maryam Ahmadi et al., in partnership with Fluidic Sciences and Sphere Bio
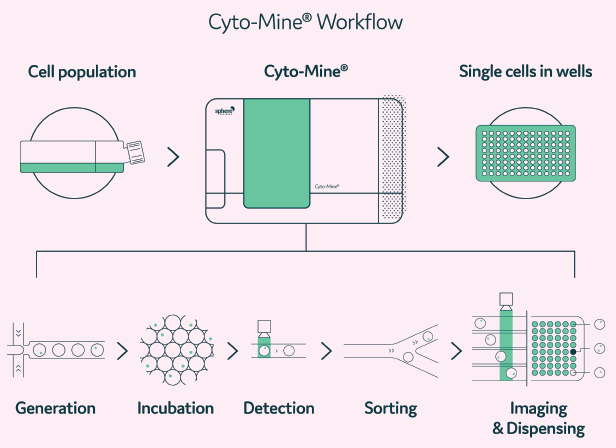
Figure 2. A Cyto-Mine® workflow. Integration of single-cell screening, sorting, isolation, and verification using a fully integrated microfluidic process. Image Credit: Maryam Ahmadi et al., in partnership with Fluidic Sciences and Sphere Bio
Table 1. Features of Cyto-Mine® and Cyto-Mine® Chroma. Source: Fluidic Sciences and Sphere Bio
| |
Cyto-Mine® |
Cyto-Mine® Chroma |
| Fully integrated |
Y |
Y |
| Automated microfluidic |
Y |
Y |
| Software & hardware control system |
Y |
Y |
| Encapsulate single cells in picodroplets |
Y |
Y |
| Selectively sort & image picodroplets |
Y |
Y |
| Ensure monoclonality |
Y |
Y |
| Dispense ‘hits’ into microtiter plate |
Y |
Y |
| Identify & isolate rare cells/cells with high productivity |
Y |
Y |
| of lasers |
1 |
4 |
| Assay multiplexing |
N |
Y |
| Reduce CLD/AbD process time |
Y |
YY |
Cyto-Mine® harnesses picodroplet technology to find rare and valuable variants within heterogeneous cell samples. The machine integrates single-cell encapsulation (generation), a fluorescence-based optical detection method followed by sorting for isolation of ‘hits,’ verification by imaging of monoclonality assurance, and single-cell dispensing into microtiter plates.
Increasing efficiency of cell line development processes with Cyto-Mine®
Cyto-Mine® increases the success rate in gene integration, clone selection, and monoclonality assurance
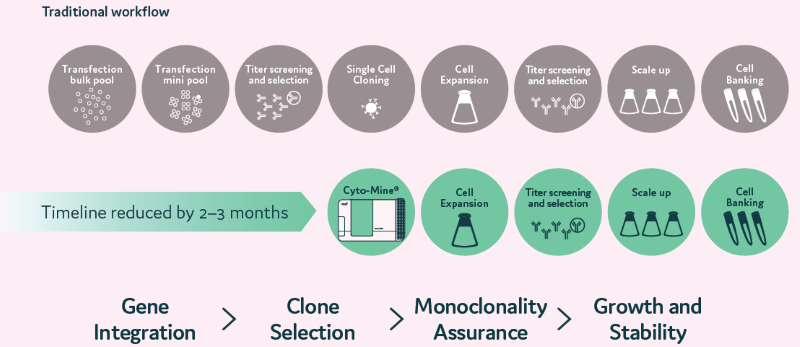
Figure 3. Traditional CLD processes are time-consuming. Cyto-Mine® reduces time and increases success rates. At a single-cell level, the clones are selected based on their productivity. Selected single cells are dispensed into microplates with image assurance on their monoclonality. Cells dispensed into microplates can be monitored for growth over time. Image Credit: Maryam Ahmadi et al., in partnership with Fluidic Sciences and Sphere Bio
Gene integration - increasing efficiency using picodroplets
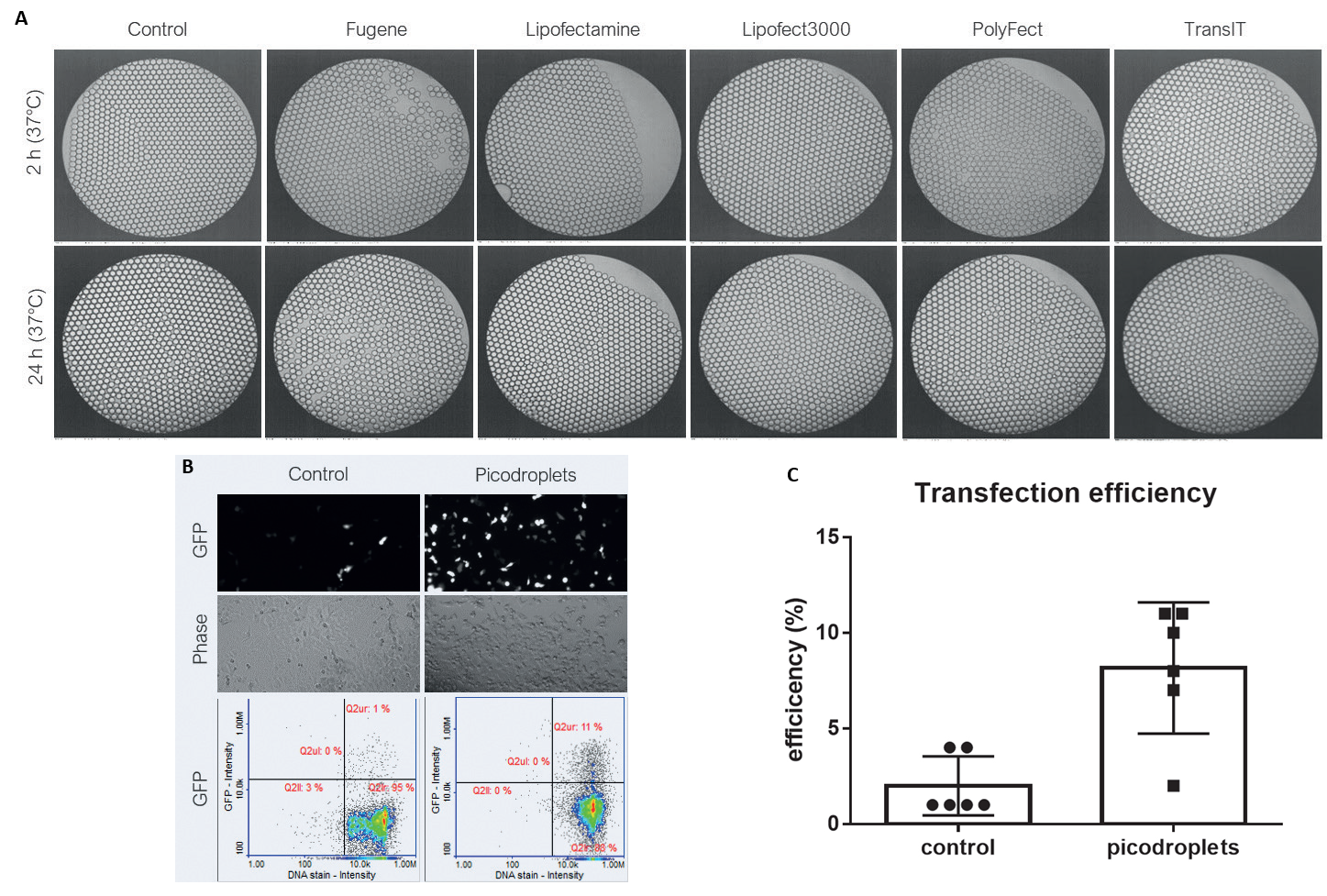
Figure 4. A) Compatibility of picodroplets with different transfection reagents. B) Cells transfected with DNA (GFP)/Lipofectamine 3000 in picodroplets C) 4 x increase in transfection efficiency in picodroplets vs. control. Image Credit: Maryam Ahmadi et al., in partnership with Fluidic Sciences and Sphere Bio
Clone selection using FRET assays
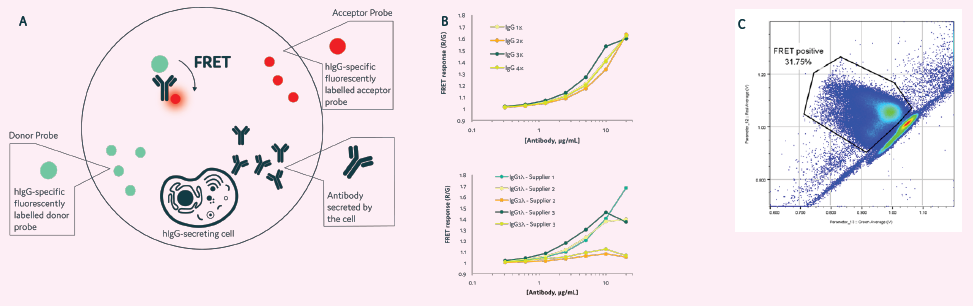
Figure 5. Clone Selection using CytoCellect®PLUS. A&B) The kit detects secreted IgG antibodies regardless of their light chain. C) The kit can be used with Cyto-Mine® to select high-producing clones, even if the IgG light chain (kappa or lambda) is unknown. Image Credit: Maryam Ahmadi et al., in partnership with Fluidic Sciences and Sphere Bio
Clone selection - using FC fusion to determine productivity
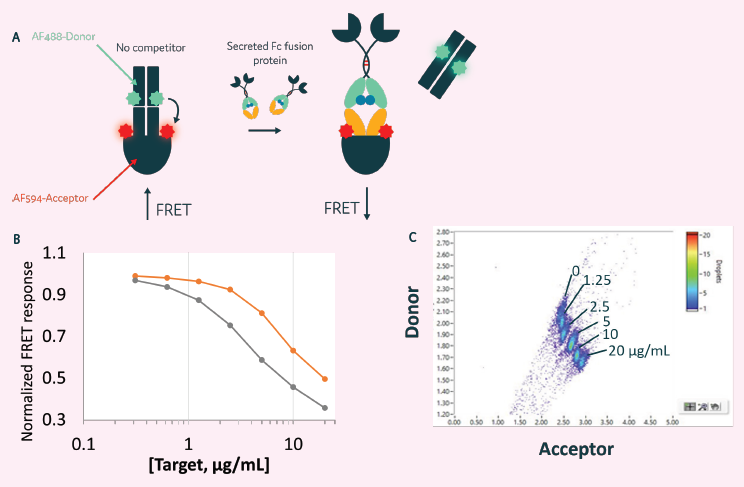
Figure 6. Clone selection for cells secreting Fc Fusion proteins. A) A quantitative competition FRET assay used to detect human Fc fusion proteins. B) Can be used to detect Fc fusion proteins in a plate reader assay format. C) Can also be used with Cyto-Mine® to detect secreted protein. Image Credit: Maryam Ahmadi et al., in partnership with Fluidic Sciences and Sphere Bio
Monoclonality assurance
- Cyto-Mine® has been specifically designed to ensure monoclonality via accurate and reliable single-cell dispensing.
- Cyto-Mine® dispenses and reports clonality of cells with an accuracy of 98.9 %.
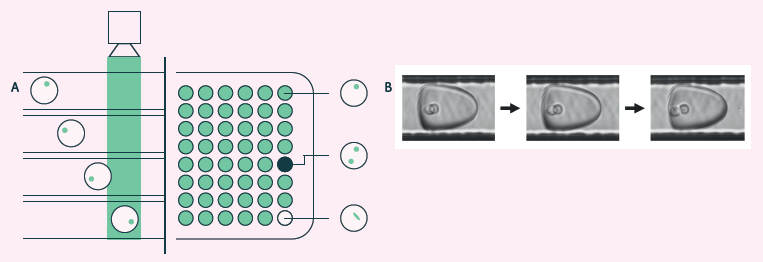
Figure 7. A) Cyto-Mine® visual verification and dispensing. Immediately before dispensing, picodroplets are brightfield-imaged to identify and record the number of cells per picodroplet and re-measured for picodroplet fluorescence. B) Brightfield multi-frame imaging avoids potential miscounting by detecting encapsulated cells at multiple timepoints as they rotate into different positions. Image Credit: Maryam Ahmadi et al., in partnership with Fluidic Sciences and Sphere Bio
Growth and stability - Maintaining outgrowth
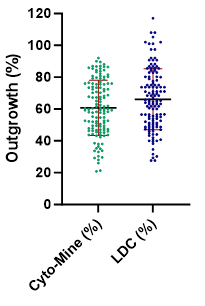
Figure 8. Cell outgrowth using Cyto-Mine® vs Limited Dilution Cloning (LDC). Image Credit: Maryam Ahmadi et al., in partnership with Fluidic Sciences and Sphere Bio
- Single cells isolated using Cyto-Mine® have high viability and show comparable growth compared to Limited Dilution Cloning
Enabling antibody discovery using Cyto-Mine®
Cyto-Mine® brings speed and simplicity to antibody discovery processes
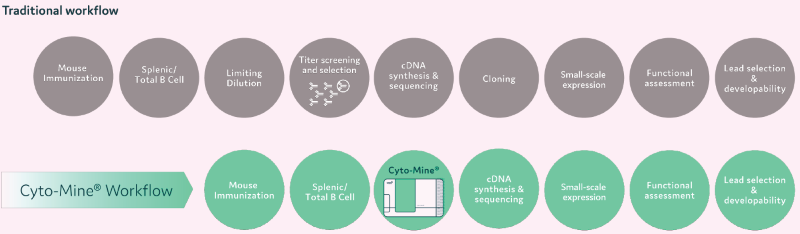
Figure 9. Antibody discovery workflow using Cyto-Mine®. Image Credit: Maryam Ahmadi et al., in partnership with Fluidic Sciences and Sphere Bio
B cell repertoire screening
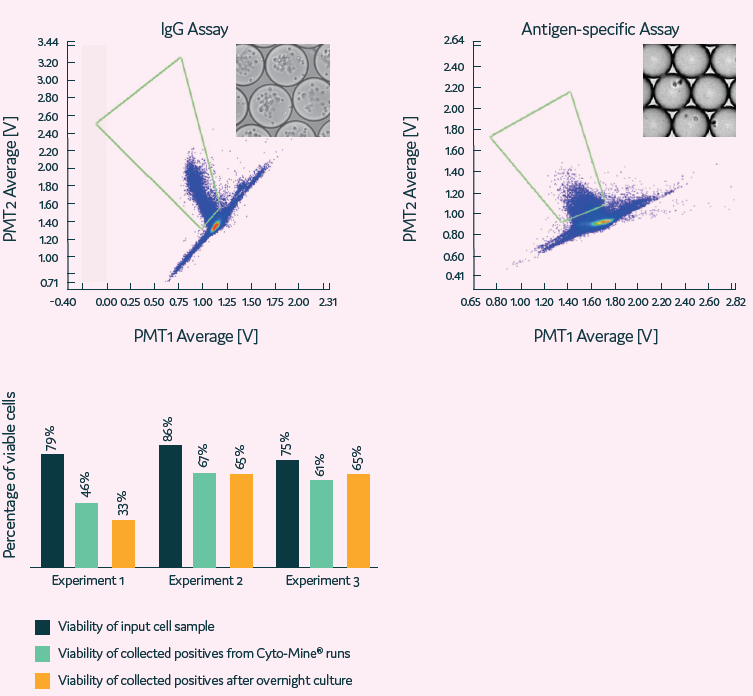
Figure 10. High-throughput single B cell screening using whole B cell repertoire. B cells are selected based on IgG and/or antigen specificity secretion. Image Credit: Maryam Ahmadi et al., in partnership with Fluidic Sciences and Sphere Bio
- Cyto-Mine® is used for high-throughput B cell screening and isolating rare cells based on their productivity.
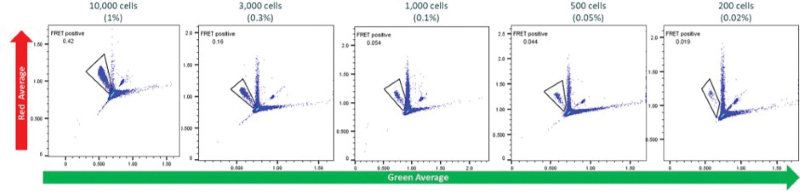
Figure 11. High sensitivity isolation of rare single cells based on their antigen specificity. Image Credit: Maryam Ahmadi et al., in partnership with Fluidic Sciences and Sphere Bio
Developing Cyto-Mine® Chroma
Going beyond gene integration
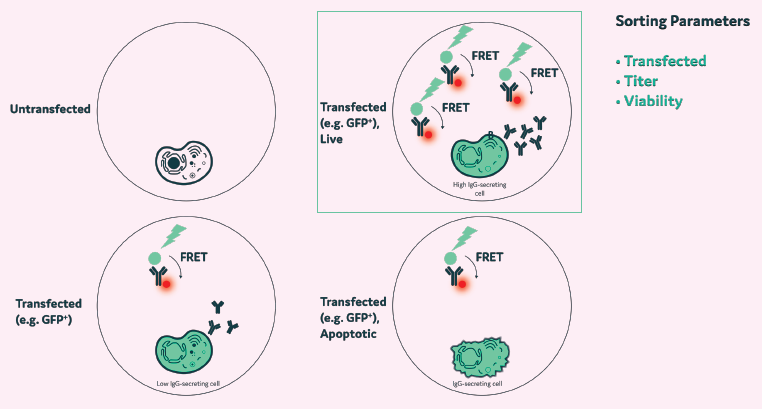
Figure 12. Multiplexing enables the isolation of clones based on the efficiency of DNA integration, viability, and titer. Image Credit: Maryam Ahmadi et al., in partnership with Fluidic Sciences and Sphere Bio
- Transfection takes place in picodroplets
- Cells are incubated for up to 72 hours, and the most productive, viable cells are isolated using Cyto- Mine® Chroma
A more efficient selection process
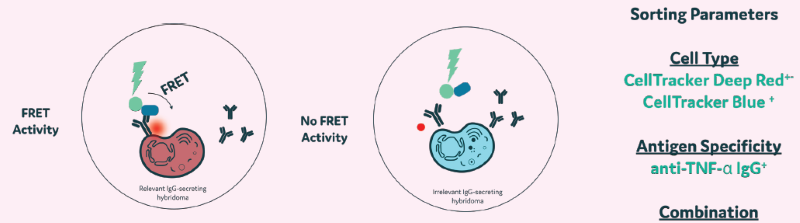
Figure 13. Example selection process. Hybridoma A: Labelled with CellTracker Blue; produces irrelevant IgG. Hybridoma B: Labelled with CellTracker Deep Red, produces an anti-TNF-alpha antibody. Image Credit: Maryam Ahmadi et al., in partnership with Fluidic Sciences and Sphere Bio
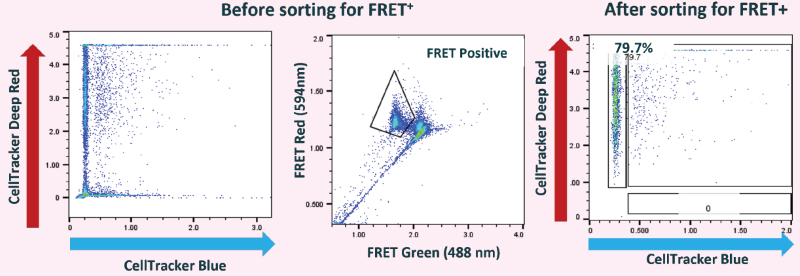
Figure 14. Single cells producing anti-TNF-alpha were detected and isolated using Cyto-Mine® Chroma (recombinant TNF-alpha was used for antigen-specific FRET assay). Image Credit: Maryam Ahmadi et al., in partnership with Fluidic Sciences and Sphere Bio
- Multiplexing assays in a single droplet improves the selection process of high-producing clones
- Cells can be imaged for viability, productivity, and antigen specificity in a single step
- Additional applications include mode of action assays involving multiple cells per droplet, where the function of a labeled cell type is evaluated in the presence of an unlabeled cell within picodroplets.
Expanding Cyto-Mine® applications: Re-injection
- Off-line generated picodroplets are injected into Cyto-Mine®
- Droplets are sorted and dispensed using Cyto-Mine®
Relevant applications include:
- Applications requiring long incubation of cells in droplets
- Applications requiring extensive manipulation during droplet generation
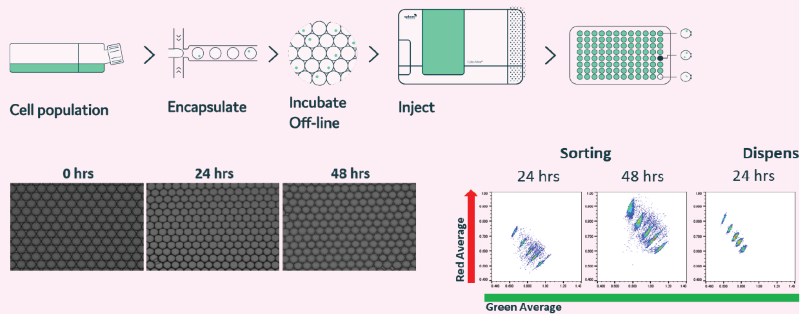
Figure 15. Off-line generated droplets are stable for prolonged periods of time and can be re-injected into Cyto-Mine®. Droplets were generated and incubated at 37 °C, 5 % CO2 for up to 48 h. (A) Microscopy images were taken at 0, 24, and 48-hour time points. (B) In the presence of FRET probes, droplets containing mouse IgG (4 different concentrations) were re-injected into Cyto-Mine®. Data shows that prolonged incubation does not affect droplet stability. Image Credit: Maryam Ahmadi et al., in partnership with Fluidic Sciences and Sphere Bio
References:
- Kaplon, H., et al. (2022). Antibodies to watch in 2022. mAbs, 14(1). https://doi.org/10.1080/19420862.2021.2014296.
- Bauer, J., et al. (2023). How can we discover developable antibody-based biotherapeutics? Frontiers in Molecular Biosciences, [online] 10, p.1221626. https://doi.org/10.3389/fmolb.2023.1221626.
- Farid, S.S., et al. (2020). Benchmarking biopharmaceutical process development and manufacturing cost contributions to R&D. mAbs, [online] 12(1), p.1754999. https://doi.org/10.1080/19420862.2020.1754999.
About Fluidic Sciences and Sphere Bio
Fluidic Sciences develops transformative in‑solution technologies for protein interaction analysis. Its flagship Fluidity One‑M instrument leverages Microfluidic Diffusional Sizing (MDS) to measure binding affinity, stoichiometry, size, and concentration without immobilization - directly in complex backgrounds such as serum, plasma, and lysate.
Sphere Bio is a brand of Fluidic Sciences. Its technology develops and manufactures single‑cell analysis and monoclonality assurance systems that enable researchers to find, analyze, and isolate the most valuable cells with speed and precision. Its proprietary picodroplet microfluidics and Cyto‑Mine® Chroma multiplexing platform power applications across antibody discovery, cell line development, cell engineering, and cell therapy.
About ELRIG (UK) Ltd.
The European Laboratory Research & Innovation Group (ELRIG) is a leading European not-for-profit organization that exists to provide outstanding scientific content to the life science community. The foundation of the organization is based on the use and application of automation, robotics and instrumentation in life science laboratories, but over time, we have evolved to respond to the needs of biopharma by developing scientific programmes that focus on cutting-edge research areas that have the potential to revolutionize drug discovery.
Comprised of a global community of over 12,000 life science professionals, participating in our events, whether it be at one of our scientific conferences or one of our networking meetings, will enable any of our community to exchange information, within disciplines and across academic and biopharmaceutical organizations, on an open access basis, as all our events are free-of-charge to attend!
Our values
Our values are to always ensure the highest quality of content and that content will be made readily accessible to all, and that we will always be an inclusive organization, serving a diverse scientific network. In addition, ELRIG will always be a volunteer led organization, run by and for the life sciences community, on a not-for-profit basis.
Our purpose
ELRIG is a company whose purpose is to bring the life science and drug discovery communities together to learn, share, connect, innovate and collaborate, on an open access basis. We achieve this through the provision of world class conferences, networking events, webinars and digital content.
Sponsored Content Policy: News-Medical.net publishes articles and related content that may be derived from sources where we have existing commercial relationships, provided such content adds value to the core editorial ethos of News-Medical.Net which is to educate and inform site visitors interested in medical research, science, medical devices and treatments.
Last Updated: Oct 25, 2025