Chimeric antigen receptor T cells are genetically modified to target specific cells; they have been used in research-based cancer treatments. Treatment is associated with numerous side effects and research is being performed on how to reduce them.
Skip to
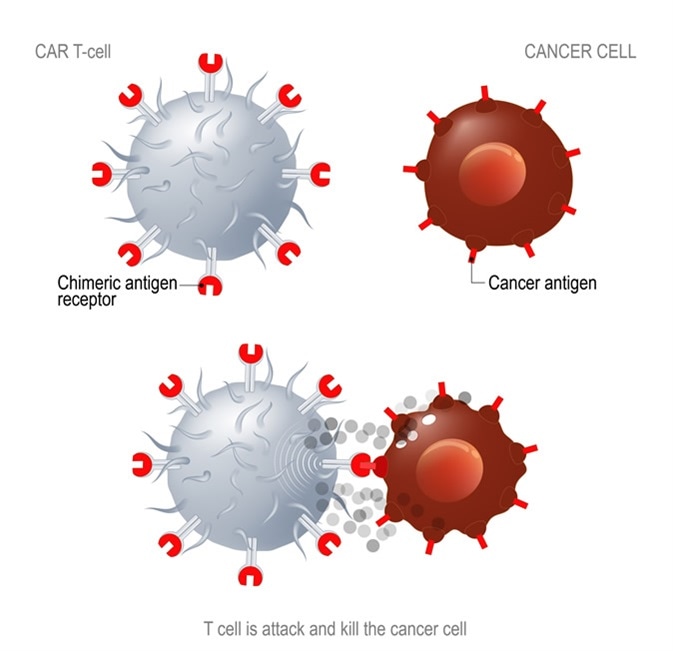
CAR T immunotherapy. Artificial T cell receptors are proteins that have been engineered for cancer therapy (killing of tumor cells). Image Credit: Designua / Shutterstock
What are Chimeric antigen receptor T cells?
Chimeric antigen receptor (CAR) T cells are patient T cells that have been genetically modified with synthetic receptors to target a surface antigen of cancerous cells. This induces an immune response that targets cancerous cells, which allows for more effective targeted cancer therapies and treatments for patients.
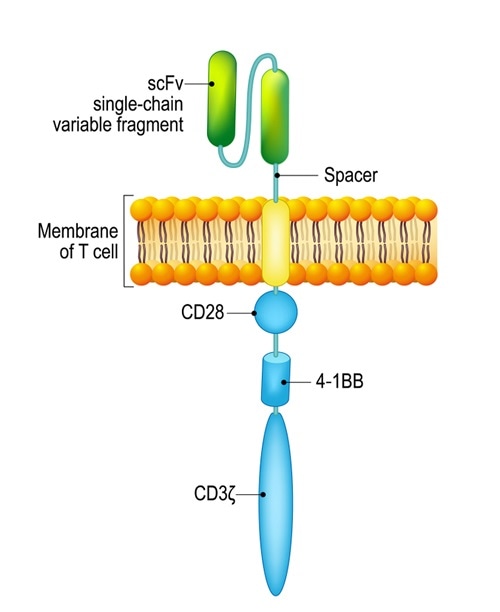
Chimeric antigen receptor T cell (CAR). Image Credit: Designua / Shutterstock
The production of CAR T cells involves the collection of peripheral blood mononuclear cells from patients, activating T cells, then the gene transfer of the desired antigen receptor. This is followed by ex vivo expansion of the CAR T cells, then end-of-process formulation, and then release testing. Once the batch passes the release testing, they can be used for research, clinical trials, and treatments.
CAR T cell therapy for cancer treatment: How it works
Clinical uses for chimeric antigen receptor T cells
The ideal target for CAR T cell therapy is cell surface markers that are exclusively expressed or overexpressed on the majority of tumor cells with very little or no expression on normal tissues. Targets for CAR T cells include:
- Overexpressed antigens such as epidermal growth factor receptor (EGFR) and human epidermal growth factor receptor 2 (HER2)
- Aberrantly glycosylated proteins such as mucin 1 (MUC1)
- Oncofetal antigens such as carcinoembryonic antigen (CEA)
- Tumor-associated stroma proteins such as fibroblast activating protein (FAP)
- Immunomodulatory antigens such as programmed death-ligand 1 (PD-L1) and certain cluster of differentiation antigens (e.g. CD19, CD22)
To date, cancers studied for treatment with these markers include lung cancer, thymic cancer, esophageal cancer, pleural mesothelioma, and certain leukemias.
Early trials used CAR T cells to target CD19-positive hematologic malignancies, such as follicular lymphoma, acute lymphocytic leukemia (ALL), and mantle cell lymphoma. The CAR T cell showed effective responses, with approximately 65-100% of ALL patients achieving complete remission. This research also led to the development of anti-CD22 CAR T cells that can be used in patients with CD22 ALL. Clinical trials are also attempting to put suicide genes in the CAR T cells that can be activated to kill them, this is effectively a molecular safety switch.
Chimeric antigen receptor T cells toxicity
A huge concern when using CAR T cells is the ‘on-target, off-tumor' toxicity which results in the destruction of normal tissues that unfortunately also express the target antigen. B-cell aplasia arises when anti-CD19 CAR T cells kill normal B lymphocytes that express CD19. This can be managed with gamma globulin replacement therapy. A case report also showed acute toxicities in a patient receiving HER2-specific CAR T cell treatments, as HER2 is expressed in low levels on various different tissues including the heart and pulmonary vasculature.
Cytokine release syndrome (CRS) arises from the activation of a large number of T cells. This leads to symptoms such as high fever, hypotension, hypoxia, and can even cause organ failure. These symptoms arise due to the production of high amounts of inflammatory cytokines such as IL-6, TNFα, and IFNγ. IL-6 blocking agents and corticosteroids can be used to manage the symptoms of severe CRS without reducing the effectiveness of the CAR T cell treatment.
CAR T cell treatments can also cause neurological symptoms including delirium, dysphasia, and seizures. The mechanisms of how CAR T cells cause these symptoms are not very well understood so treatment options for them are very limited, however, in most cases these symptoms resolve themselves within a few days with no external intervention.
Chimeric antigen receptor T cells safety profiles
CAR T cells can have some severe side-effects and toxicities, therefore improving CAR T cell safety is an extremely important topic of research. As previously mentioned, there has been research into programming suicide genes into these CAR T cells, this will help to reduce the toxicity and allow for very controlled treatments.
Another strategy that is used increase the specificity of CAR T cells is to split the signals required to activate the T cells between two CAR molecules, this will require two separate antigens on the surface of the cancerous cells to be recognized, which increases the specificity of the treatment.
Further Reading
Last Updated: May 14, 2019