This article is based on a poster originally authored by Qiang Xia, Yanan Zhao, Tiejun Bing, Charlie Yin, and Joby Jenkins.
Drug safety is one of the most important considerations throughout the pharmaceutical industry, with organizations across the sector recognizing drug safety as a core aspect of drug development.
Potential adverse drug reactions (ADRs) must be identified as early as possible, meaning advanced safety pharmacology panels are integral to drug development.
ICE Bioscience has developed the ICESTP Safety Panel 90 to offer drug developers a comprehensive secondary safety pharmacology assessment.
This powerful new tool is designed to improve drug safety assessments by evaluating a drug’s interaction with a wide range of targets related to the cardiovascular system, metabolism, central nervous system, and immune system.
The ICESTP Safety Panel 90 features 138 assays, incorporating dose-response screening and single-point screening with a functional assay format, such as automatic patch clamp, HTRF, and FLIPR calcium flow.
This article outlines how SPT Labtech’s firefly® automated liquid handling platform was chosen for its high-throughput sample processing capabilities, to streamline the safety assessment of a 90-target panel and improve the efficiency and accuracy of a drug safety evaluation.
All-in-one liquid handling with firefly®
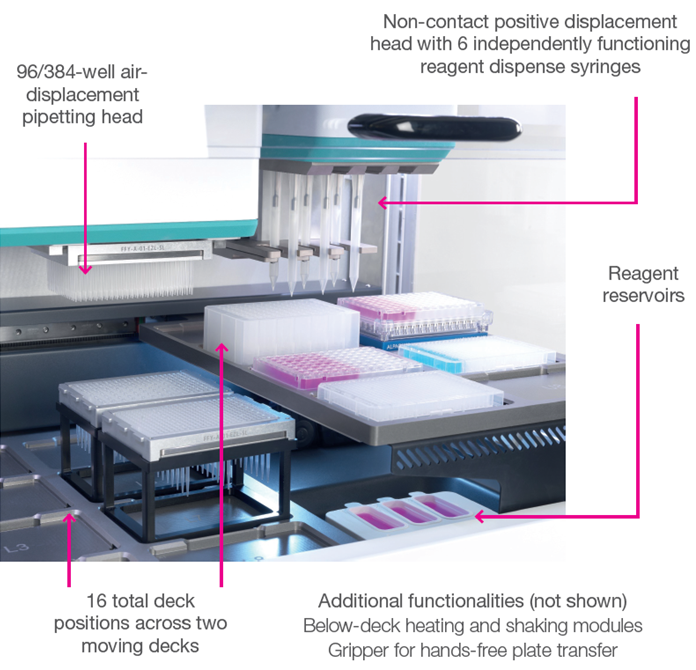
Image Credit: SPT Labtech
Methods
A powerful automated workflow was used to support the ICESTP Safety Panel 90.

Image Credit: SPT Labtech
Compound serial dilution was achieved by dispensing 20 µL of DMSO using the firefly® dispense head. A total of 10 μL of the compound was then transferred and mixed with 20 μL DMSO to complete a 3-fold serial dilution. This was performed using the firefly® pipetting head.
The compound transfer was achieved by transferring 100 nL of the diluted compound to an assay plate via Echo.
To ensure efficient reagent transfer, 2.5 μL substrate, and 2.5 μL enzyme were added with the dispense head of firefly® in a 384-well plate, followed by a 1-hour incubation period. Next, 5 μL of detection buffer was added with the firefly® dispense head.
Results were analyzed via BMG reader or FLIPR to ensure proper signal detection.
Pergolide tested in ICESTP Safety Panel 90
The dopamine receptor agonist Pergolide is widely used in the treatment of conditions such as Parkinson’s disease. It has been linked to an increased risk of cardiac valvulopathy, however, prompting its withdrawal from the Canadian and United States markets in 2007.
The off-target profile of Peroglide was tested before utilizing the off-target representation to elucidate its ADR mechanisms. This work aimed to better explain its drug-target-ADR correlations, offering valuable information for other tasks related to safety prediction.
This early safety assessment protocol has the potential to steer a more rational drug development process and better enable the discovery of safe compounds.

Figure 1. Related class of 90 targets. Image Credit: SPT Labtech
Results
Pergolide showed low selectivity in the ICESTP Safety Panel 90. It was observed that 12 targets demonstrated over 50% activation, with two targets showing over 50% inhibition at a concentration of 10 µM (Figure 2).
Figure 3 shows EC50/IC50 dose-response curves generated using 10 dose levels. These off-target effects increase the risk of cardiac valvulopathy.

Figure 2. Pergolide activation or inhibition % with functional assay format in ICESTP Safety Panel 90. Image Credit: SPT Labtech

Figure 3. Pergolide activation or inhibition using dose-response with function assay format in ICESTP Safety Panel 90 (display of partial data). Image Credit: SPT Labtech
Conclusion
Drug clinical safety can be improved through in vitro secondary pharmacology profiling, as highlighted by a notable decline in drug off-target promiscuity over the past decade. This has also correlated with a reduction in severe adverse events for drugs currently on the market.
The ICESTP Safety Panel 90 leverages functional assay formats with dose-response modes and single-point screening. firefly® from SPT Labtech is an ideal tool for conducting compound serial dilution and precious reagent addition, enabling the acquisition of high-quality, reliable data when employed in the functional assay for ICESTP Safety Panel 90.
firefly® is supporting the work of ICE Bioscience, allowing the company to be one of the world’s first contract research organizations (CROs) to offer full-functional and curve-fitting for secondary safety pharmacology assessment, covering over 90 targets.
Advantages of ICESTP Safety Panel 90. Source: SPT Labtech
| Functional assay format |
Dose-response screening |
| Ability to distinguish agonist and antagonist |
Robust and reproducible data |
| Ability to detect allosteric pharmacology |
Ability to highlight partial agonists |
| Close to the physiological situation (e.g. 1 mM ATP used in kinase assays) |
Potential to highlight solubility issues |
| Provides for a more stringent analysis, resulting in fewer follow-up studies |
Quickly correlate with in vivo exposure values |
| Efficient and time-saving |
Efficient and time-saving |
References and further reading
- Bowes, J., et al. (2012). Reducing safety-related drug attrition: the use of in vitro pharmacological profiling. Nature Reviews Drug Discovery, 11(12), pp.909–922. https://doi.org/10.1038/nrd3845.
- Finan, C., et al. (2017). The druggable genome and support for target identification and validation in drug development. Science Translational Medicine, 9(383), p.eaag1166. https://doi.org/10.1126/scitranslmed.aag1166.
- Lynch, J.J., et al. (2017). Potential functional and pathological side effects related to off-target pharmacological activity. Journal of Pharmacological and Toxicological Methods, 87, pp.108–126. https://doi.org/10.1016/j.vascn.2017.02.020.
- Sutherland, J.J., Dimitar Yonchev, Fekete, A. and László Urbán (2023). A preclinical secondary pharmacology resource illuminates target-adverse drug reaction associations of marketed drugs. Nature Communications, 14(1). https://doi.org/10.1038/s41467-023-40064-9.
- Valentin, J.-P., et al. (2018). In vitro secondary pharmacological profiling: An IQ-DruSafe industry survey on current practices. Journal of pharmacological and toxicological methods, [online] 93, pp.7–14. https://doi.org/10.1016/j.vascn.2018.07.001.
- Brennan, R.J., et al. (2024). The state of the art in secondary pharmacology and its impact on the safety of new medicines. Nature Reviews Drug Discovery, (online) 23(7), pp.525–545. https://doi.org/10.1038/s41573-024-00942-3.
- Clarke, C.E. and Speller, J. (1999). Pergolide for levodopa-induced complications in Parkinson’s disease. Cochrane Database of Systematic Reviews. https://doi.org/10.1002/14651858.cd000235.
Acknowledgments
Produced from materials originally authored by Qiang Xia, Yanan Zhao, and Tiejun Bing from ICE Bioscience; and Charlie Yin and Joby Jenkins from SPT Labtech.
About SPT Labtech

We Design and Manufacture Robust, Reliable and Easy-to-Use Solutions for Life Science
We enable life scientists through collaboration, deep application knowledge, and leading engineering to accelerate research and make a difference together. We offer a portfolio of products within sample management, liquid handling, and multiplexed detection that minimize assay volumes, reduce material handling costs and put the discovery tools back in the hands of the scientist.
At the heart of what we do
Many of our innovations have been born out of the desire to create solutions to existing customer problems; and it’s this ethos that drives SPT Labtech’s R&D efforts. Our strengths come from the trust our customers have with us to develop truly unique, automated technologies to meet their needs. We combine cutting edge science with first-rate engineering to put customers at the heart of everything we do.
A problem-solving state of mind
The substantial breadth of expertise within our company enables us to be involved in the full life cycle of our products from the initial design concept, mechanical and software engineering and prototyping, to final manufacture and sale. These qualities allow us to offer the best possible technical and mechanical support to all the equipment that we supply, hence maintaining excellent client relationships.
SPT Labtech Company Overview
Sponsored Content Policy: News-Medical.net publishes articles and related content that may be derived from sources where we have existing commercial relationships, provided such content adds value to the core editorial ethos of News-Medical.Net which is to educate and inform site visitors interested in medical research, science, medical devices and treatments.