Drug discovery workflows benefit from the use of assay miniaturization as it reduces the number of reagents and samples required. Low-volume assays also contribute to a reduction in costs.
The miniaturization of assays often involves a limitation on the ability to pipette small volumes of various liquids in an accurate and reproducible way.
Icagen Tucson Innovation Center (Tucson, AZ, USA) is a specialized pharmaceutical partnering organization that focuses on early drug discovery. The organization assessed the use of the mosquito® LV instrument (from SPT Labtech) for low-volume qRTPCR and the serial dilution of drug compounds.
This article discusses how automated liquid handling can enhance the quality of these applications in drug discovery.
Miniaturizing drug discovery applications with mosquito LV
Reducing assay volumes conserves the compound used, minimizes the number of consumables required and, because compounds are diluted initially in DMSO, ensures compound solubility
However, these benefits can only be realized achieved with accurate and reproducible liquid handling.
SPT Labtech’s mosquito HV (0.5 – 5 µL) and mosquito LV (25 nL - 1.2 µL) are automated either 8- or 16-channel liquid handlers, as shown in Figure 1.

Figure 1. mosquito LV (8- or 16- channel) liquid handler. Image Credit: SPT Labtech
Case study 1: Miniaturizing qRTPCR reactions
Low-volume workflows for qRTPCR were tested, dispensing reagents and samples serially to ensure sufficient sensitivity and linearity of cDNA dilutions.
Methods
The qRTPCR standard curve reactions were set up in triplicate with either of the following:
- 300 nL cell lysates from a rat s16 Schwann cell line that has been treated with a selection of compounds.
- Dilutions of cDNA (target and housekeeping genes at 1, 0.5, 0.25, and 0,125x dilutions).
Master Mix was added to create final volumes of 2-3 µL, respectively.
The required volumes of reagents and samples were dispensed into 384 well plates with the mosquito LV, and subsequent qRTPCR gene analysis was conducted using a Roche LightCyler, as displayed in Figure 2.
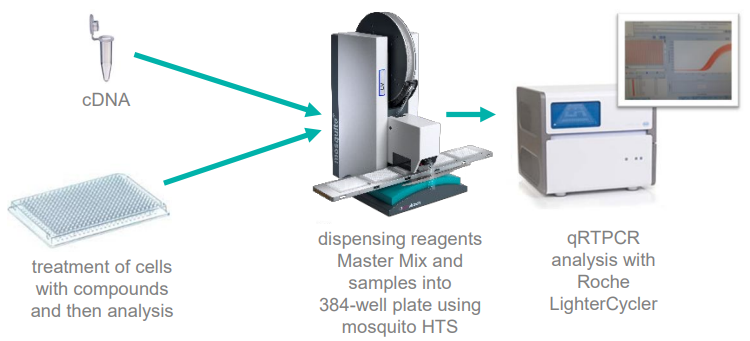
Figure 2. Miniaturized qRTPCR workflows using mosquito HTS. Image Credit: SPT Labtech
Results
The results for both experiments displayed reproducible and highly accurate pipetting with CVs of less than 3%, as well as a linear relationship between dilutions, as shown in Figure 3 and Figure 4.
IC50 measurements determined from the low-volume qRTPCR reaction of cell lysates (derived from drug treatment of cells) were similar to that of established techniques.
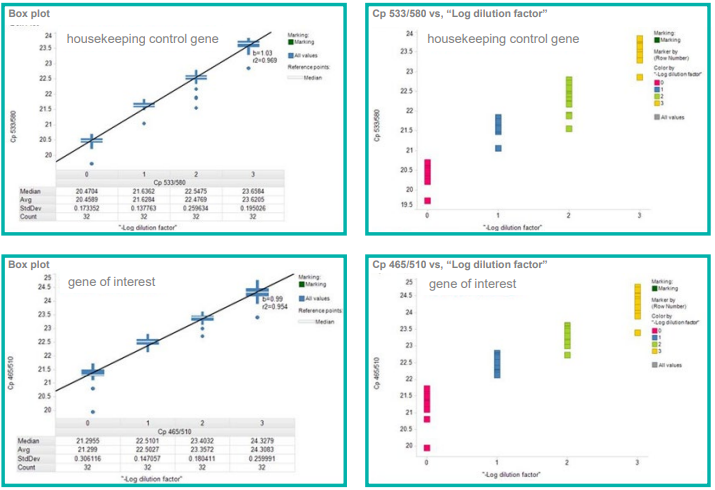
Figure 3. Validation of low-volume cDNA standard curves prepared in 2 µL final volumes using mosquito LV. 0, 1, 2 and 3 represent dilutions of cDNA of 1x, 0.5x, 0.25x and 0.125x observed CVs for technical repeats were < 3%. The graphs demonstrate cDNA maintained linearity of signal. Image Credit: SPT Labtech
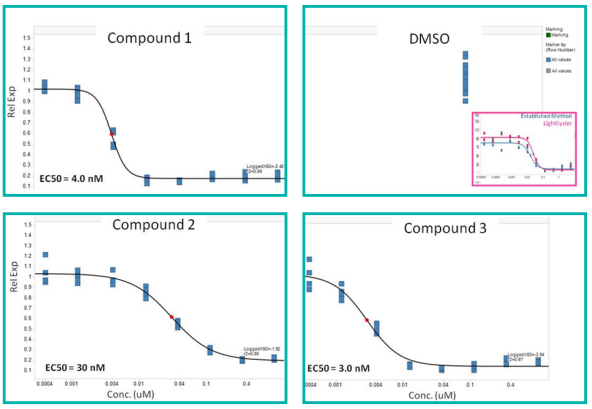
Figure 4. Evaluation of qRTPCR gene analysis of 300 nL cell lysates prepared in 3 µL final volumes using mosquito LV observed CVs for technical repeats were < 3%. The calculated IC50s were comparable to established methods (inset). Image Credit: SPT Labtech
Case study 2: Low-volume dilution curves for drug compounds
Icagen Tucson Innovation Center regularly analyzes serial dilutions of lead optimization compounds in simultaneous cellular and biochemical assays for a rare disease project.
The assays are devised to recognize the activation of compounds in both assays at the same time, on replicate plates and employing the same compound dilution plate.
Errors in manipulation or reproducibility are reduced as assays are run on the same day and using the same dilution plate.
Method
A 7-point 2x dilution curve of compounds plus DMSO (final volume 500 nL) was prepared in a 384-well plate with the mosquito HTS.
These prepared plates were further diluted in reaction buffer with a Biomek FX and dispensed to replicate daughter plates for analysis of compounds in molecular and cellular assays.
A BMG Labtech Clariostar was employed to detect a fluorescent product generated by the reaction of compounds with the substrate, as displayed in Figure 5.
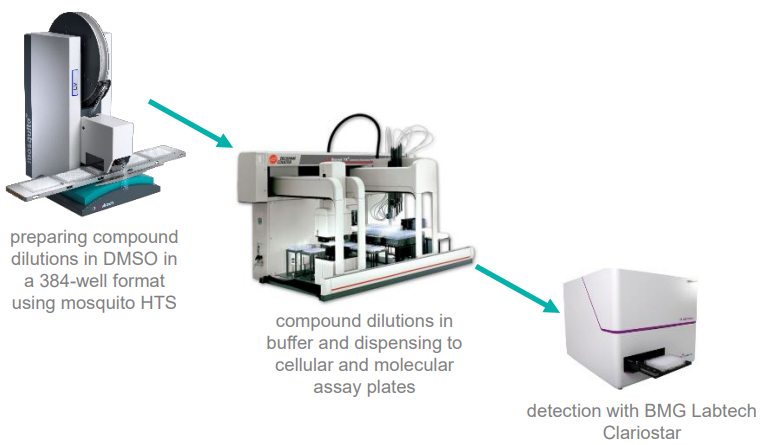
Figure 5. Low-volume drug dilution workflow using mosquito HTS. Image Credit: SPT Labtech
Results
The results revealed that compound consumption can be reduced to as little as 0.5 µL per assay. Decreased consumables used for replicate assays and plates lead to decreased costs as well as reduced errors.
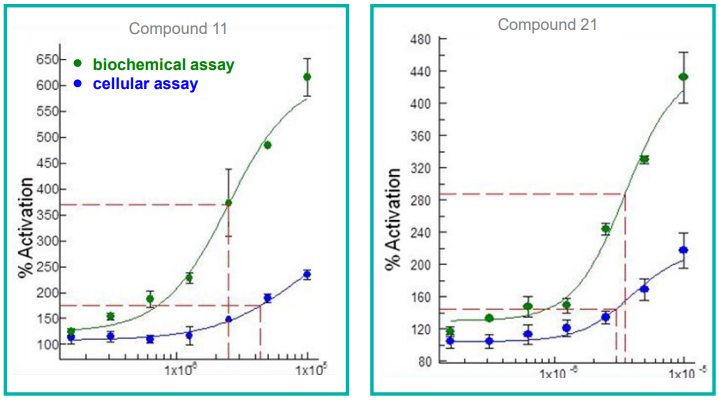
Figure 6. Concentration curves for compound activity in both biochemical and cellular assays. Image Credit: SPT Labtech
Conclusions
The mosquito LV from SPT Labtech was validated for two distinct drug discovery applications. These are as follows:
Low-volume drug dilution for use in multiple assays
- Ensures solubility of compounds
- Decreased consumption of compounds
- Decreased use of consumables
- Enables assay-ready plate preparation
- Reduced errors in reproducibility by running cellular and biochemical assays with the same drug dilution plate
qRTPCR low-volume workflow
- cDNA titration maintained the linearity of signal
- Low CVs for technical replicates of <3% in 2 µL working volume
- IC50s were determined in line with established methods
- Detection of suitable gene expression with only 300 nL of cell lysates
Acknowledgments
Produced from materials originally authored by Margaret Kenney,2 Archi Joardar,2 Vasudeo Badarinarayana,2 and Joby Jenkins1 at 1SPT Labtech Ltd, Melbourn Science Park, UK, and 2Icagen, Tucson, AZ, USA.
About SPT Labtech
We design and manufacture robust, reliable and easy-to-use solutions for life science
We enable life scientists through collaboration, deep application knowledge, and leading engineering to accelerate research and make a difference together. We offer a portfolio of products within sample management, liquid handling, and multiplexed detection that minimize assay volumes, reduce material handling costs and put the discovery tools back in the hands of the scientist.
At the heart of what we do
Many of our innovations have been born out of the desire to create solutions to existing customer problems; and it’s this ethos that drives SPT Labtech’s R&D efforts. Our strengths come from the trust our customers have with us to develop truly unique, automated technologies to meet their needs. We combine cutting edge science with first-rate engineering to put customers at the heart of everything we do.
A problem-solving state of mind
The substantial breadth of expertise within our company enables us to be involved in the full life cycle of our products from the initial design concept, mechanical and software engineering and prototyping, to final manufacture and sale. These qualities allow us to offer the best possible technical and mechanical support to all the equipment that we supply, hence maintaining excellent client relationships.
Sponsored Content Policy: News-Medical.net publishes articles and related content that may be derived from sources where we have existing commercial relationships, provided such content adds value to the core editorial ethos of News-Medical.Net which is to educate and inform site visitors interested in medical research, science, medical devices and treatments.