What is diabetes?
What is epigenetics? How is it implicated in human disease?
The current understanding of the causes of type 2 diabetes
The epigenetics of type 2 diabetes
Implications for treatment
References
Further reading
Epigenetics, the study of changes in gene expression that are not caused by alterations in the DNA sequence, has emerged as a key player in the development and severity of type 2 diabetes.
 Epigenetics and diabetes. Image Credit: Maya Kruchankova/Shutterstock.com
Epigenetics and diabetes. Image Credit: Maya Kruchankova/Shutterstock.com
Recent studies have shown that the interplay between the epigenome, environmental factors, and the gut microbiota influences the onset and progression of the disease. Understanding the complex mechanisms of epigenetics may lead to new treatment approaches.
What is diabetes?
Diabetes refers to a metabolic disorder characterized by high glucose levels in the blood. It affects 463 million people worldwide, and it is expected to increase to 700 million by 2045.
Diabetes is classified into three categories based on their pathophysiology: type 1, type 2, and gestational diabetes. Type 2 Diabetes (T2D) is the most prevalent, accounting for over 90% of all cases of diabetes.
While genetic susceptibility is a critical factor in T2D development and severity, non-genetic aspects such as diet and physical activity also play a significant role. Recently, epigenetic mechanisms and their interplay with the microbiota have emerged as a new area of exploration in basic and clinical research for T2D.
What is epigenetics? How is it implicated in human disease?
Epigenetics denotes heritable changes in gene expression and activity (in the progeny of individuals or cells ) as well as long-term, stable alterations in the transcriptional potential of a cell which are not always heritable.
The field of epigenetics investigates how endogenous and exogenous factors such as diet, gut microbiota, environment and medication impact the human phenotype without altering the DNA sequence but instead by modifying gene transcription.
This modulation of gene expression is achieved through alterations in DNA methylation, histone modifications, chromatin remodeling, and non-coding RNA mechanisms, all of which affect the epigenome. Histone modifications (acetylation and methylation) are critical in activating and repressing gene transcription. Therefore, the epigenome is the collective epigenetic information present in a cell.
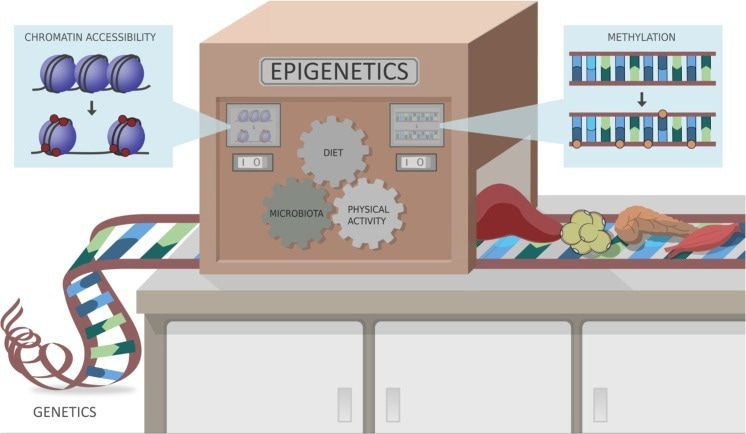 Study: Epigenetic Markers and Microbiota/Metabolite-Induced Epigenetic Modifications in the Pathogenesis of Obesity, Metabolic Syndrome, Type 2 Diabetes, and Non-alcoholic Fatty Liver Disease. Image Credit: Stols-Gonçalves at al. (2019)
Study: Epigenetic Markers and Microbiota/Metabolite-Induced Epigenetic Modifications in the Pathogenesis of Obesity, Metabolic Syndrome, Type 2 Diabetes, and Non-alcoholic Fatty Liver Disease. Image Credit: Stols-Gonçalves at al. (2019)
Epigenetics plays a crucial role in the development and progression of several human diseases. Epigenetic modifications have a considerable impact on gene expression patterns, leading to disease onset or progression.
These changes can directly cause disease or intensify its susceptibility in response to environmental factors. For example, epigenetic modifications in cancers have been implicated in the growth and survival of tumor cells through DNA methylation and histone modification patterns.
Epigenetic changes have also been implicated in cardiovascular diseases, diabetes, and autoimmune disorders. In certain circumstances, these changes can persist across multiple generations, contributing to disease development in families or populations.
These epigenetic modifications have been associated with a variety of diseases, ranging from cancer, cardiovascular diseases, diabetes, neurodegenerative and psychiatric disorders, autoimmune diseases, allergies, Fragile X syndrome, Angelman syndrome, Prader-Willi syndrome, Rubinstein-Taybi syndrome and Huntington's disease.
These epigenetic changes are not the only cause of these pathologies; they are the result of interactions between environmental and genetic factors.
The current understanding of the causes of type 2 diabetes
Type 2 diabetes is a multi-factorial disease, caused by a combination of genetic, environmental, and lifestyle factors. Insulin resistance is a key risk factor, occurring when the body's cells become less sensitive to the insulin hormone that regulates blood sugar levels.
Having a family history of diabetes, as well as specific gene variants, such as TCF7L2, ABCC8, KCNQ1, KCNJ11, PDX1, MC4R, GLUT4, and SORCS1, increases the odds of developing type 2 diabetes.
Obesity, being overweight, and a sedentary lifestyle are major risk factors for developing insulin resistance and subsequently type 2 diabetes. Several medical conditions, such as polycystic ovary syndrome and hormonal disorders are linked to type 2 diabetes risk. In addition, certain medications, such as antipsychotics and corticosteroids, have been shown to increase the likelihood of developing type 2 diabetes.
The epigenetics of type 2 diabetes
Initial studies analyzing the DNA methylation of candidate genes for T2D (INS, PDX1, PPARGC1A, and GLP1R) in human pancreatic islets from T2D and non-diabetic patients found an increase in DNA methylation and a decrease in the expression of these key genes. This finding was associated with impaired insulin secretion. Additionally, high glucose and glycated hemoglobin (HbA1c) appear to directly increase DNA methylation of these genes.
Later on, Illumina's Infinium arrays allowed the simultaneous analysis of methylation of multiple CpG sites. Methylation analyses of 27,000 and 450,000 CpG sites in pancreatic islets from T2D and non-diabetic donors showed altered DNA methylation in 1,649 CpG sites corresponding to 843 genes in T2D cases compared to controls.
102 of these genes also had differential gene expression. A decrease in DNA methylation of CDKN1A, PDE7B, and SEPT9 caused an increase in gene expression in T2D islets, having direct negative effects on their transcriptional activity. The overexpression of these genes resulted in lower glucose-stimulated insulin secretion.
New methods like WGBS for DNA methylation analysis, ChIP-seq for histone modifications, and ATAC-seq for chromatin accessibility analysis have allowed the study of multiple epigenetic modifications related to T2D development.
There is evidence that the gut microbiota has an effect on epigenetic regulation in T2D. The genera Bifidobacterium, Bacteroides, Faecalibacterium, Akkermansia and Roseburia are negatively associated with T2D, while Ruminococcus, Fusobacterium, and Blautia are positively associated.
Roseburia and Faecalibacterium species are SCFAs-producing bacteria, such as butyrate and propionate, which are important in epigenetic modulations of Histone deacetylases (HDACs).
Butyrate also plays a role in Treg cell differentiation through the acetylation of noncoding regions of the FOXP3 locus. Changes in the gut microbiota and epigenetic DNA methylation of TLR2 and TLR4 have been observed in individuals with T2D.
Implications for treatment
Epigenetics plays an important role in the development and progression of type 2 diabetes, and there is growing interest in developing epigenetic-based therapies for its treatment. There are different epigenetic treatments for T2D.
Drugs that target histone methylations have been proposed, including inhibitors of chromatin-associated epigenomic writers (e.g. EZH2), erasers (e.g. LSD1), and readers (e.g. BRD4). Metformin has been shown to reverse the H3K36me2 mark in pre-diabetic and diet-induced obesity mouse models, and it directly targets the H3K27me3 demethylase KDM6A/UTX.
Lactobacillus supplementation can modulate the histone methylation profile in insulin resistance (IR) by preventing the methylation and demethylation of H3K79me2 and H3K27me3, respectively.
Directly administering beneficial bacteria or probiotics, making changes in the diet, and increasing physical activity have been shown to influence epigenetic modifications and may be essential for T2D prevention and management.
References
- Gibney ER, et al. (2010). Epigenetics and gene expression. Heredity, 105(1). doi:10.1038/hdy.2010.54. https://www.nature.com/articles/hdy201054
- Gurung M, et al. (2020). Role of gut microbiota in type 2 diabetes pathophysiology. EBioMedicine, 51. doi:10.1016/j.ebiom.2019.11.051. https://pubmed.ncbi.nlm.nih.gov/31901868/
- Ling C, et al. (2019). Epigenetics in human obesity and type 2 diabetes. Cell Metabolism. 29(5), pp. 1028–1044. doi:10.1016/j.cmet.2019.03.009. https://pubmed.ncbi.nlm.nih.gov/30982733/
- Sharma M, et al. (2020). The epigenetic connection between the gut microbiome in obesity and diabetes. Frontiers in Genetics, 10. doi:10.3389/fgene.2019.01329. https://www.frontiersin.org/articles/10.3389/fgene.2019.01329/full
- Stols-Gonçalves D, et al. (2019). Epigenetic markers and microbiota/metabolite-induced epigenetic modifications in the pathogenesis of obesity, metabolic syndrome, type 2 diabetes, and non-alcoholic fatty liver disease. Current Diabetes Reports, 19(8). doi:10.1007/s11892-019-1151-4. https://link.springer.com/article/10.1007/s11892-019-1151-4
- Yang Y, et al. (2022). Epigenetics and beyond: targeting histone methylation to treat type 2 diabetes mellitus. Frontiers in Pharmacology, 12. doi:10.3389/fphar.2021.807413. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC8788853/
- Epigenetic mechanisms in psychiatric disorders (2021). [Online] Psych Scene Hub. https://psychscenehub.com/psychinsights/epigenetic-mechanisms-in-psychiatric-disorders-major-depression-psychosis-and-addiction/ (Accessed on July 2023).
Further Reading
Last Updated: Aug 7, 2023