What is cell signaling?
How cell signaling goes wrong
The impact of dysregulated cell signaling
Therapeutic targets and future directions
References
Further reading
Cell signaling is crucial for cells to communicate and function correctly. Disruptions in these pathways, caused by genetic mutations or environmental factors, can lead to uncontrolled cell growth, improper immune responses, or errors in development. These dysregulations are the basis for diseases like cancer, diabetes, and autoimmune disorders.
Image Credit: Billion Photos/Shutterstock.com
What is cell signaling?
Cell signaling involves the different stages in which cells communicate with each other and their environment. It is a complex process involving a series of steps that allow cells to receive, process (transduction), and respond to signals.1
In order to communicate with each other, cells require key components, such as receptors, signaling molecules, target proteins, as well as scaffold proteins and second messenger molecules.1
Cell signaling receptors are proteins on the cell surface or inside the cell that bind to specific signaling molecules.1 These receptors can identify and translate different external stimuli, such as mechanical, chemical, or electrical stimuli, into a chemical language that the cell can understand and respond to.1
These mechanisms are called mechanotransduction, electrotransduction, and chemotransduction, respectively. Signaling molecules are molecules that carry signals from one cell to another.1 They can be hormones, neurotransmitters, growth factors, or other molecules.1
Target proteins reside inside the cell and are activated or deactivated by the signaling pathway, leading to a specific cellular response.1 In the same context, a variety of other molecules are required for the proper progression or development of a signaling pathway. These are second messengers and scaffold proteins.1
Second messengers, as the name implies, carry the information received by the specific receptor but amplify it so that the response can spread throughout the entire cell, and responses can be more efficient and rapid.1
The Fundamentals of Disease: What Does Inflammation Do to the Body?
Scaffold proteins are another key component, as they help to assemble protein complexes and reduce physical distances between crucial proteins in the signaling pathways.1 They participate in constructing these macromolecular complexes needed for a proper signaling response.1
How cell signaling goes wrong
Cell signaling is crucial for maintaining proper bodily functions. However, disruptions to these signaling pathways, also called dysregulation, can contribute to various conditions like cancer, neurodegenerative disorders, and autoimmune diseases.
There are different causes of signaling dysregulation. Mutations can lead to malfunctioning signaling proteins, disrupting signal transmission. For example, mutations in genes encoding receptor tyrosine kinases (RTKs), such as epidermal growth factor receptor (EGFR) or fibroblast growth factor receptor (FGFR), are implicated in cancer development.2 Mutations in the RAS gene, a key regulator of cell growth, are also common in various cancers.3
Pathogens can hijack cellular signaling pathways for their benefit, disrupting normal cellular processes.4,5 For instance, the bacterium Helicobacter pylori alter signaling pathways in stomach cells, contributing to ulcer formation.4 This also happens in infections caused by parasites like Trypanosoma cruzi.5
Exposure to toxins, pollutants, radiation, or other environmental factors can interfere with cell signaling.6 In addition to cancers, impaired signaling also contributes to neuronal dysfunction in diseases like Alzheimer's and Parkinson's.7 In Alzheimer's, altered processing of amyloid precursor protein disrupts signaling pathways crucial for neuronal survival.7
Dysregulation of cytokine signaling, which mediates immune responses, contributes to inflammation and tissue damage in autoimmune diseases like rheumatoid arthritis.8 It is important to note that a primary response against a pathogen can drive autoimmune disorders.9
The impact of dysregulated cell signaling
Dysregulated cell signaling has profound consequences for cellular behavior and overall health, leading to a range of pathological conditions.
Proper cell signaling tightly controls cell growth and division. Dysregulation can tip the balance, generating uncontrolled proliferation. Overactivation of pathways driven by growth factors like epidermal growth factor (EGF) or fibroblast growth factor (FGF) can fuel excessive cell division. Mutations in genes encoding receptors for these growth factors (EGFR and FGFR) are commonly observed in cancers.2
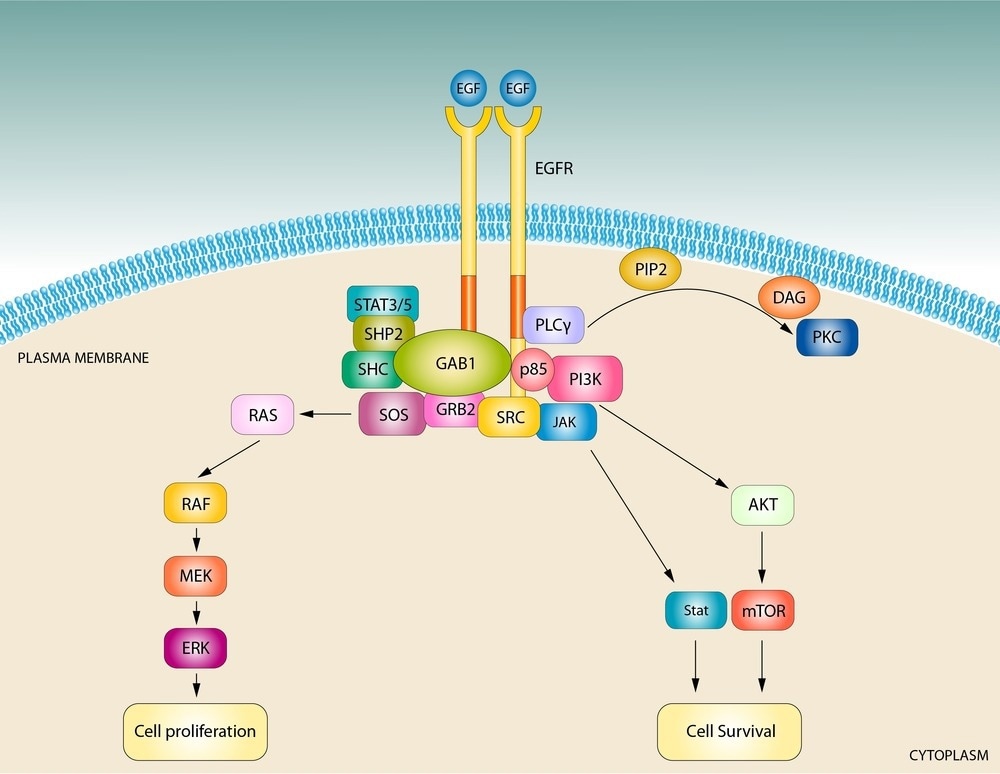 The EGF signaling pathway regulates cell growth, proliferation, differentiation, and survival through the activation of EGFR and downstream cascades like RAS/RAF/MEK/ERK and PI3K/AKT. Dysregulation, caused by mutations, overexpression, or impaired regulation, is linked to diseases such as cancer, psoriasis, and fibrosis. In cancers like non-small cell lung cancer and glioblastoma, aberrant EGF signaling drives uncontrolled growth and therapy resistance. Targeted therapies, including tyrosine kinase inhibitors and monoclonal antibodies, have been developed to address these disruptions, though resistance remains a significant challenge.
The EGF signaling pathway regulates cell growth, proliferation, differentiation, and survival through the activation of EGFR and downstream cascades like RAS/RAF/MEK/ERK and PI3K/AKT. Dysregulation, caused by mutations, overexpression, or impaired regulation, is linked to diseases such as cancer, psoriasis, and fibrosis. In cancers like non-small cell lung cancer and glioblastoma, aberrant EGF signaling drives uncontrolled growth and therapy resistance. Targeted therapies, including tyrosine kinase inhibitors and monoclonal antibodies, have been developed to address these disruptions, though resistance remains a significant challenge.
Dysregulation of cell cycle checkpoints, which ensure orderly progression through the cell division cycle, can allow cells with DNA damage to proliferate, contributing to genomic instability and tumor development.10
Additionally, cells have a programmed mechanism called apoptosis to eliminate damaged or non-functional cells.11 Dysregulation of these pathways allows damaged cells to survive, hindering the homeostatic cellular balance and potentially leading to the development of different diseases.11
A common feature of cancer cells is the acquisition of mutations that inactivate pro-apoptotic proteins or upregulate anti-apoptotic signals, allowing them to evade programmed cell death and continue proliferating.11
However, impaired apoptosis can lead to other disorders.11 For example, it contributes to the accumulation of misfolded proteins and cellular debris in neurodegenerative diseases like Alzheimer's and Parkinson's.11
Cells constantly encounter various stressors, and proper signaling is crucial for mounting appropriate responses.12 Dysregulation can compromise cellular adaptation to stress, leading to dysfunction and disease.12
Cells generate reactive oxygen species (ROS) as byproducts of metabolism.12 Dysregulation of antioxidant defense mechanisms can lead to oxidative stress, damaging cellular components and contributing to aging and various diseases.12
In the same context, chronic inflammation, often driven by dysregulated cytokine signaling, can contribute to tissue damage and the development of chronic diseases like autoimmune disorders and cardiovascular diseases.13
Therapeutic targets and future directions
As dysregulated cell signaling drives numerous diseases, there is an urgent need for targeted therapies.14 Developing drugs that modulate specific signaling pathways holds immense promise for treating conditions like cancer, neurodegenerative diseases, and autoimmune disorders.1
For example, inhibitors of receptor tyrosine kinases (RTKs) have shown efficacy in cancers driven by aberrant RTK signaling.15 Nonetheless, the future of medicine lies in personalized approaches, tailoring treatments based on an individual's genetic and molecular profile.14 This could involve identifying specific mutations driving disease and selecting drugs that precisely target those dysregulated pathways.14
Future research will increasingly focus on harnessing the power of omics technologies such as genomics or proteomics.16,17 By elucidating complex signaling networks and identifying novel therapeutic targets, scientists aim to develop therapies tailored to an individual's unique molecular makeup.16,17
This approach promises to maximize efficacy while minimizing side effects.16,17 Through genomics, they can identify specific mutations that drive disease, enabling the selection of drugs that precisely target these dysregulated pathways.16,17
Proteomics can further refine this approach by identifying protein biomarkers that predict drug response or disease progression.16 The integration of these advances with a deeper understanding of cell biology will pave the way for truly personalized therapies and revolutionize the treatment of a wide range of diseases.17
References
- Su, J. et al. Cell-cell communication: new insights and clinical implications. Signal Transduct Target Ther 9, 196 (2024). https://doi.org/10.1038/s41392-024-01888-z
- Paul, M. K. & Mukhopadhyay, A. K. Tyrosine kinase - Role and significance in Cancer. Int J Med Sci 1, 101-115 (2004). https://doi.org/10.7150/ijms.1.101
- Simanshu, D. K., Nissley, D. V. & McCormick, F. RAS Proteins and Their Regulators in Human Disease. Cell 170, 17-33 (2017). https://doi.org/10.1016/j.cell.2017.06.009
- Alzahrani, S. et al. Effect of Helicobacter pylori on gastric epithelial cells. World J Gastroenterol 20, 12767-12780 (2014). https://doi.org/10.3748/wjg.v20.i36.12767
- Volpini, X. et al. Trypanosoma cruzi Exploits Wnt Signaling Pathway to Promote Its Intracellular Replication in Macrophages. Front Immunol 9, 859 (2018). https://doi.org/10.3389/fimmu.2018.00859
- He, K. et al. Environmental endocrine disruptor-induced mitochondrial dysfunction: a potential mechanism underlying diabetes and its complications. Front Endocrinol (Lausanne) 15, 1422752 (2024). https://doi.org/10.3389/fendo.2024.1422752
- Hampel, H. et al. The Amyloid-beta Pathway in Alzheimer's Disease. Mol Psychiatry 26, 5481-5503 (2021). https://doi.org/10.1038/s41380-021-01249-0
- Alunno, A., Carubbi, F., Giacomelli, R. & Gerli, R. Cytokines in the pathogenesis of rheumatoid arthritis: new players and therapeutic targets. BMC Rheumatol 1, 3 (2017). https://doi.org/10.1186/s41927-017-0001-8
- Qiu, C. C., Caricchio, R. & Gallucci, S. Triggers of Autoimmunity: The Role of Bacterial Infections in the Extracellular Exposure of Lupus Nuclear Autoantigens. Front Immunol 10, 2608 (2019). https://doi.org/10.3389/fimmu.2019.02608
- Visconti, R., Della Monica, R. & Grieco, D. Cell cycle checkpoint in cancer: a therapeutically targetable double-edged sword. J Exp Clin Cancer Res 35, 153 (2016). https://doi.org/10.1186/s13046-016-0433-9
- Favaloro, B., Allocati, N., Graziano, V., Di Ilio, C. & De Laurenzi, V. Role of apoptosis in disease. Aging (Albany NY) 4, 330-349 (2012). https://doi.org/10.18632/aging.100459
- Butterfield, D. A. & Halliwell, B. Oxidative stress, dysfunctional glucose metabolism and Alzheimer disease. Nat Rev Neurosci 20, 148-160 (2019). https://doi.org/10.1038/s41583-019-0132-6
- Stergioti, E. M., Manolakou, T., Boumpas, D. T. & Banos, A. Antiviral Innate Immune Responses in Autoimmunity: Receptors, Pathways, and Therapeutic Targeting. Biomedicines 10 (2022). https://doi.org/10.3390/biomedicines10112820
- Ho, D. et al. Enabling Technologies for Personalized and Precision Medicine. Trends Biotechnol 38, 497-518 (2020). https://doi.org/10.1016/j.tibtech.2019.12.021
- Tomuleasa, C. et al. Therapeutic advances of targeting receptor tyrosine kinases in cancer. Signal Transduct Target Ther 9, 201 (2024). https://doi.org/10.1038/s41392-024-01899-w
- Duarte, T. T. & Spencer, C. T. Personalized Proteomics: The Future of Precision Medicine. Proteomes 4 (2016). https://doi.org/10.3390/proteomes4040029
- Olivier, M., Asmis, R., Hawkins, G. A., Howard, T. D. & Cox, L. A. The Need for Multi-Omics Biomarker Signatures in Precision Medicine. Int J Mol Sci 20 (2019). https://doi.org/10.3390/ijms20194781
Further Reading
Last Updated: Dec 2, 2024