This article is based on a poster originally authored by Jennifer Yin, Kimiko Kearney, Abigail Keller, Rodriel Bautista, Chelsea Pratt, Dipika Gurnani and Nathan Sepulveda.
Gene therapy is an advanced technique that treats disease by introducing DNA into human cells. Adeno-associated viruses (AAV), which consist of a DNA payload enclosed inside a capsid shell, are regularly employed as vectors for this purpose.
However, some capsids can be empty following production, meaning that they lack the DNA payload. Full capsids contribute to the therapeutic effect; however, empty capsids provide no clinical benefits and increase the risk of an adverse immune response.
To address this issue, Bio-Rad Laboratories has developed a highly accurate kit to measure the percentage of full capsids in both purified and crude AAV samples.
Kit procedure
First, samples were prepared by removing external DNA using DNase digestion. The next step was binding, during which antibody-oligos bind to AAV capsid.
Image Credit: Bio-Rad Laboratories
Oligos were then joined by a splint and ligated together in a process called proximity ligation. Finally, ligated oligo pairs were amplified and detected in Droplet Digital PCR (ddPCR), together with a standard assay to detect the AAV genome.
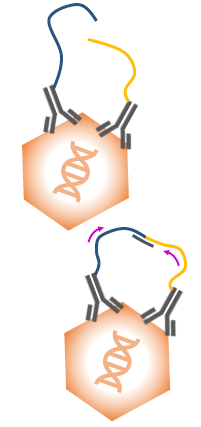
Image Credit: Bio-Rad Laboratories
Quantification
A single test was used to measure genome titer, capsid titer, and the percentage of full capsids without the use of external standard curves. This was achieved by calibrating the capsid assay using the relationship (linkage) between the single-positive genome droplets and the double-positive capsid + genome droplets.
A background signal was then measured by testing a negative control containing no AAV.
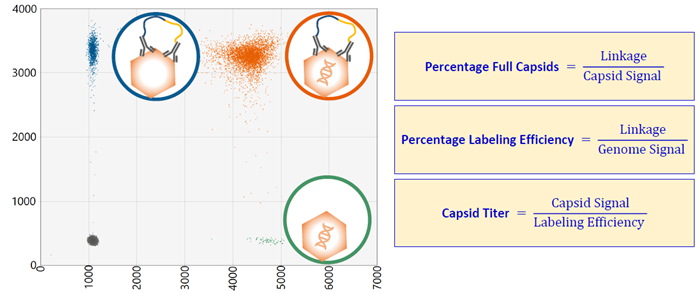
Image Credit: Bio-Rad Laboratories
Dynamic range
Genome titer, capsid titer, and the percentage of full capsids can be calculated across a broad dynamic range. Due to the linkage calibration, demonstrated by the coefficient of variation (CV), percentage full measurements are more precise than titer measurements.
Source: Bio-Rad Laboratories
| Sample |
Genome
Titer |
Genome
Titer,
%CV |
Capsid
Titer |
Capsid
Titer,
%CV |
Percent
Full, % |
Percent
Full, %CV |
| 1 |
5.4E+13 |
8.0 |
7.1E+13 |
9.3 |
76.4 |
1.4 |
| 2 |
5.1E+12 |
9.3 |
6.7E+12 |
9.6 |
76.7 |
0.6 |
| 3 |
4.8E+11 |
8.3 |
6.3E+11 |
8.5 |
76.5 |
0.1 |
| 4 |
4.4E+10 |
18.3 |
5.7E+10 |
18.4 |
76.8 |
0.8 |
| 5 |
4.8E+09 |
14.0 |
6.2E+09 |
14.5 |
76.9 |
0.9 |
n = 3
Sample types
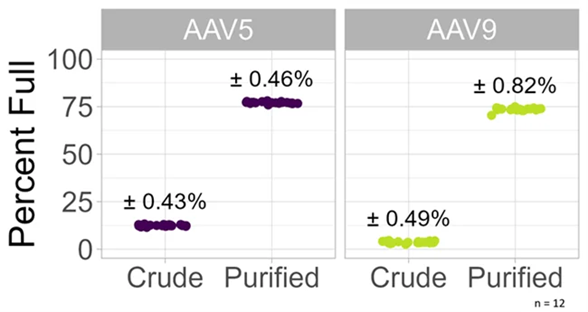
Image Credit: Bio-Rad Laboratories
In the study presented in this article, the Vericheck ddPCR Empty-Full Capsid Kit was tested in 12 replicates, with both purified AAV and crude cell lysate containing both AAV5 and AAV9. The precision was excellent in both cases. No additional steps were required to measure crude samples.
Empty-to-full linearity
To create five samples across the measuring range, an AAV sample containing mostly filled capsids was mixed with empty capsids at various ratios and tested with 24 replicates each. Slope and r2 were close to 1.
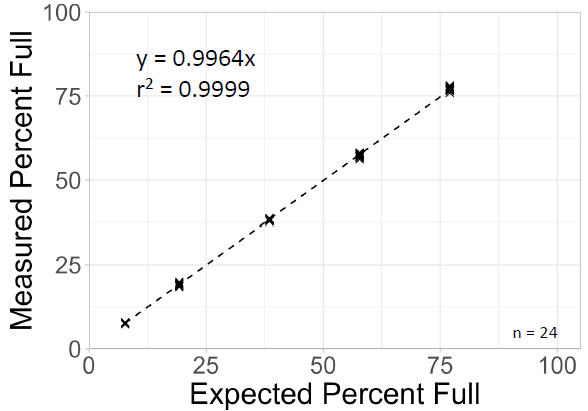
Image Credit: Bio-Rad Laboratories
Partial genomes
An ITR2 genome reference assay is included in the kit; however, any internalized target can be used, allowing poorly packaged genes to be made visible. AAV containing the genome ITR2-CMVe-CMVp-eGFP-SV40-ITR2 were tested with multiple assays, revealing different packaging patterns.
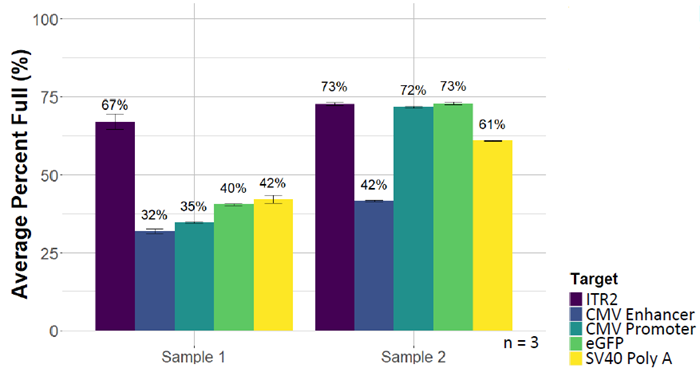
Image Credit: Bio-Rad Laboratories
Comparison with analytical ultracentrifugation
Source: Bio-Rad Laboratories
| Serotype |
AUC, % |
Droplet Digital PCR |
| ITR2, % |
KanR, % |
Rep, % |
E2A, % |
| 5 |
69.8 |
57.8 |
4.9 |
0.14 |
0.01 |
| 9 |
81.8 |
71.8 |
2.6 |
0.11 |
0.01 |
n = 3
Unlike contents-blind methods, such as AUC, which overestimate the number of therapeutic capsids by calling contaminants full, Droplet Digital PCR is specific to capsid contents.
In the samples presented above, capsids containing residual plasmid DNA were labeled as full by AUC but negative by Droplet Digital PCR. Additional residual DNA from host cell DNA may account for further differences.
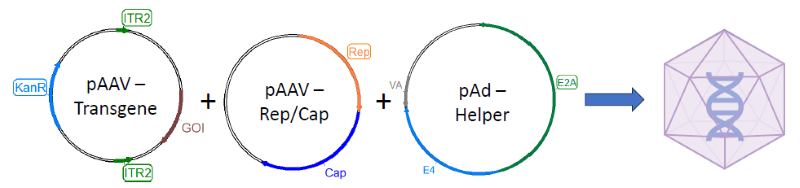
Image Credit: Bio-Rad Laboratories
Conclusions
The Vericheck ddPCR Empty-Full Capsid Kit can be used by scientists to measure genome titer, capsid titer and the percentage of full capsid with high precision, providing a more comprehensive assessment of AAV samples than previously possible.
Kits targeting AAV5 and AAV9 serotypes were launched on the QX200™, QX600 ™ and QX ONE Droplet Digital PCR systems in September 2024.
Image Credit: Bio-Rad Laboratories
Acknowledgments
Produced from materials originally authored by Jennifer Yin, Kimiko Kearney, Abigail Keller, Rodriel Bautista, Chelsea Pratt, Dipika Gurnani and Nathan Sepulveda from Bio-Rad Laboratories.
About Bio-Rad Laboratories
For over six decades, Bio-Rad has provided the healthcare industry with innovative and useful products that help life science researchers accelerate the discovery process and medical diagnostic labs obtain faster, better results.
Bio-Rad is among the top five life science companies in the world, providing instruments, software, consumables, reagents, and content for the areas of cell biology, gene expression, protein purification, protein quantitation, drug discovery and manufacture, food safety and environmental quality testing, along with science education. Our products and solutions are based on technologies to separate, purify, identify, analyze, and amplify biological materials such as antibodies, proteins, nucleic acids, cells, and bacteria.
As a leading global provider of in-vitro diagnostics supplies, our diagnostic products and systems leverage a broad range of technologies and deliver high-value clinical information in the blood transfusion, diabetes monitoring, autoimmune, and infectious disease testing markets. These products are used to support the diagnosis, monitoring, and treatment of diseases and other medical conditions.
Bio-Rad is the world leader in clinical quality control products, services, and information systems, products that ensure the accuracy and validity of clinical test results.
Sponsored Content Policy: News-Medical.net publishes articles and related content that may be derived from sources where we have existing commercial relationships, provided such content adds value to the core editorial ethos of News-Medical.Net which is to educate and inform site visitors interested in medical research, science, medical devices and treatments.
Last Updated: Nov 15, 2024