Bio-Rad Laboratories, Inc., a global leader of life science research and clinical diagnostic products, has published new findings on the generation and characterization of drug-target complex-specific antibodies for pharmacokinetic (PK) analysis of biotherapeutics.
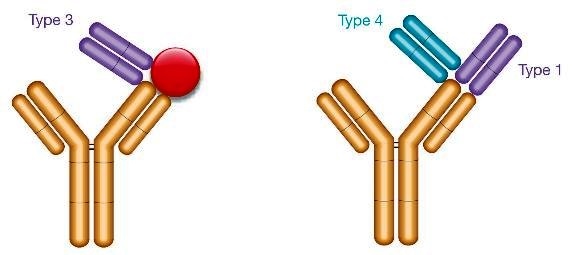
The paper published in the journal mAbs discusses antibodies that recognize the drug only when bound to its target. Called Type 3 antibodies, these antibodies differ from other anti-idiotypic antibodies that specifically detect free antibody drug by binding the paratope of the drug (Type 1) or total drug by binding outside the paratope of the drug (Type 2).
PK assays form part of the totality of evidence required for approval of an original biologic or biosimilar drug, and anti-idiotypic antibodies are critical reagents used in these types of ligand binding assays (LBAs). By describing the generation and characterization of Type 3 specificities for the development of LBAs, the authors demonstrate the advantages of these antibodies as tools for drug quantification.
The paper describes the successful generation of Type 3 antibodies directed against several approved antibody drugs using Bio-Rad’s innovative custom antibody generation service, based on the Human Combinatorial Antibody Library (HuCAL®) technology and Cys-Display®, a modified phage display method. This recombinant production ensures a consistent and secure batch-to-batch supply, which is important for assay reproducibility.
Using Type 3 antibodies, Bio-Rad’s team demonstrate increased sensitivity and specificity across several assay formats and quantify monovalent antibody fragments such as ranibizumab, which is difficult to achieve with commonly used LBAs like bridging assays.
Additionally, the team introduce a derivative of the Type 3 specificity, termed Type 4, providing an alternative when the drug target is not easily available or costly to produce, or when the selection of Type 3 is not possible.
Drug development relies on ligand binding assays, and the robustness, accuracy and reproducibility of these assays depends on the quality of critical reagents used.
This paper is important in characterizing drug-target complex-specific reagents as useful tools in those assays, demonstrating several advantageous properties, to ultimately improve the accuracy of conclusions and accelerate the drug development process.”
Stefan Harth, R&D Team Leader, Custom Antibodies, Bio-Rad, and lead author on the paper
The reagents discussed in the paper represent a valuable addition to the ligand binding assay toolbox, and the reagents offer bioanalysts options for more sophisticated PK assay design to support biotherapeutic development.
The original Type 3 and Type 4 reagents enable simple and robust assays that support the development of simplified rapid tests for therapeutic drug monitoring.”
Amanda Turner, Bio-Rad Product Manager, Life Science Group